Recent advances in the pathogenesis, diagnosis, and treatment of sepsis-associated encephalopathy
Rui Wang and Wanda Bi have contributed equally to this work.
Abstract
Sepsis is a life-threatening organ dysfunction syndrome caused by the host's dysregulated response to infection. The leading causes of death in critically ill patients are sepsis-associated encephalopathy (SAE), respiratory dysfunction, circulatory dysfunction, and other multi-organ dysfunctions. SAE is among the most common serious complications of sepsis and is associated with a poor prognosis and long-term cognitive dysfunction. Its clinical manifestations vary, and there are still no unified diagnostic criteria. The incidence of SAE varies from 9% to 71% in critically ill patients due to therapeutic interventions such as sedation, mechanical ventilation, and muscle relaxants. Advances in medical technology have significantly increased the survival rate of patients with sepsis, but up to 21% now experience long-term sequelae or cognitive impairment. The lack of specific early diagnostic and treatment methods leads to increased SAE-associated mortality and complications in patients, which also impose heavy economic burdens. This article reviews the pathogenesis and diagnostic methods of SAE and progress in its treatment, aiming to reduce the mortality and hospitalization lengths of patients with SAE and improve their survival rate and quality of life through early detection, diagnosis, and effective treatment.
Key points
What is already known about this topic?
-
Sepsis arises from the host's dysregulated response to infection. Among its severe complications, sepsis-associated encephalopathy (SAE) is notable, marked by a grim prognosis and enduring cognitive impairments. The clinical presentation of SAE varies, exacerbated by the lack of standardized diagnostic criteria. The dearth of precise early diagnostics and targeted treatments compounds mortality rates and complicating factors in patients with SAE, imposing substantial economic burdens.
What does this study add?
-
This article comprehensively reviews the intricate mechanisms implicated in SAE, encompassing disruptions in the blood-brain barrier, glial cell hyperactivation, neurotransmitter alterations, and mitochondrial dysfunction. It also critically assesses potential diagnostic and therapeutic modalities for SAE, underscoring the need to further explore their underlying mechanisms to facilitate advances in novel diagnostic techniques and treatment strategies.
1 INTRODUCTION
Sepsis and its complications are among the most common causes of death in intensive care units. During the progression of sepsis, the brain is involved in regulating not only the immune system but also vulnerable organs. The incidence of SAE ranges from 9% to 71%, with a higher incidence in patients with complications associated with liver, kidney, or multi-organ dysfunction.1 SAE is a high-risk factor for death and long-term disabling cognitive impairment in patients with sepsis.2 Its core clinical feature is diffuse brain dysfunction that cannot be located and can cause acute and chronic cognitive dysfunction. Delirium is an important clinical manifestation and may be the first symptom. Its main clinical feature is an altered level of consciousness, and patients with mild cases can experience reduced attention and alertness, anxiety, and delirium, which can progress to drowsiness and coma in patients with severe SAE. Long-term cognitive dysfunction, including dysfunctions in memory, attention, verbal fluency, and executive function, greatly impacts survivors' quality of life.
Several studies have shown that SAE has a particularly significant impact on children. After discharge, they may have neurodevelopmental and behavioral disorders in areas such as language, intelligence quotient, emotion, and adaptability; in particular, delayed language and intelligence development and irritability and shock often occur during the course of SAE. Furthermore, learning and cognition are significantly affected in children with prolonged sedation.3
Mortality from sepsis has significantly decreased with improvements in medical standards, but up to 21% of patients now experience long-term sequelae or cognitive impairment.4 Due to the lack of effective preventive and management measures for the nervous system after the onset of sepsis, SAE poses a significant threat to human health, leading to a substantial economic and social burden globally.5 The World Health Organization has designated SAE a key health focus area.6 Moreover, early diagnosis and intervention are particularly crucial for patients with SAE.
2 SAE PATHOGENESIS
While the exact mechanism of brain dysfunction remains incompletely understood, the pathophysiology of SAE is known to be multifactorial. Recent studies have shown that up to 70% of patients with sepsis develop SAE.7-11 One of the main causes of brain damage during sepsis is the significantly increased expression of proinflammatory cytokines.12, 13 In addition, the activation of microglia and astrocytes causes changes in cerebral homeostasis14, 15 (Figure 1). Neuroinflammation increases metabolic demands, leading to oxidative stress and mitochondrial dysfunction, the production of reactive oxygen species (ROS), and the induction of proapoptotic signals that affect glial cells, neurons, and the structure of the blood-brain barrier (BBB).16 Since the BBB is a highly selective barrier between the brain and the periphery, changes in BBB integrity impair healthy brain function.17, 18 Metabolic and hemodynamic changes also precede cognitive disorders and structural changes in the white and gray matter of the brain.19-21 In the pathological process of sepsis, changes in cerebral metabolism may be a key factor leading to encephalopathy.22 Once the etiology of SAE is unclear, treatment options are limited, and a single treatment strategy, such as patient sedation, is unsatisfactory because the complex situation is unresolved. Therefore, relevant research is urgently needed to reveal the molecular mechanism of SAE pathogenesis (Figure 2).
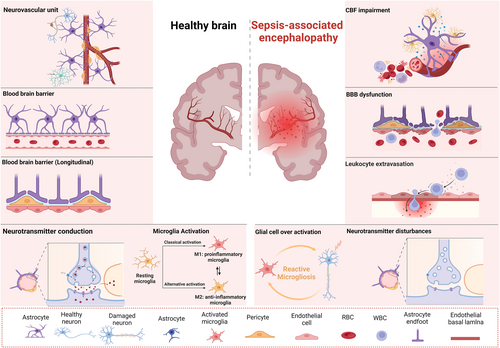
Schematic of the pathophysiological processes in SAE. Alterations in cerebral perfusion and microcirculation lead to neuronal hypoxia and apoptosis, blood cell destruction, and vascular endothelium damage. Loss of BBB integrity allows circulating monocytes, neutrophils, and proinflammatory cytokines to enter the brain parenchyma. Microglia proliferate, become reactivated, and undergo functional and morphological changes. M1 microglia (proinflammatory) and M2 microglia (anti-inflammatory) maintain the homeostasis of the central nervous system. Neuroinflammation also impacts neurons and synaptic transmission, leading to neurotransmitter system dysfunctions. BBB, blood-brain barrier; SAE, septic-associated encephalopathy.
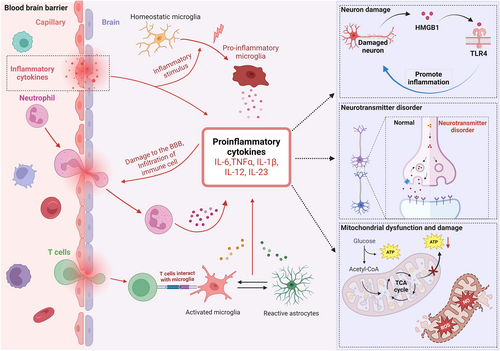
Proposed pathological mechanisms through which cytokines trigger SAE. An excessive immune response during sepsis leads to the accumulation of cytokines such as IL-6, TNF-α, IL-1β, IL-12, and IL-23 in the serum, which may induce BBB disruption, facilitating the penetration of inflammatory cells and cytokines into the brain. Cytokines in the brain activate astrocytes and microglia, which then release additional cytokines, exacerbating BBB damage. Some invading T cells activate microglia to release proinflammatory cytokines, which activate astrocytes and release proinflammatory cytokines. During this process, cytokine release increases in a cascade, leading to neurotransmitter disorders, neuronal mitochondrial dysfunction, and HMGB1-TLR4-dependent inflammatory neuron damage. BBB, blood-brain barrier; SAE, septic-associated encephalopathy; TLR4, toll-like receptor 4.
2.1 BBB damage and dysfunction
The BBB is composed of endothelial cells, astrocyte foot processes, and adventitial cells. Under physiological conditions, the BBB controls cerebrovascular blood flow by regulating the microenvironment of the nervous system, reducing harmful factors in brain tissue, and providing a relatively stable internal environment for brain tissue. In the early stage of sepsis, the immune inflammatory response is overactivated, and many cytokines are released, causing the activation and dysfunction of cerebrovascular endothelial cells.10, 23 In sepsis, the tight junctions of vascular endothelial cells open, and the expression of tight junction proteins is reduced.24 Moreover, cytokines and endotoxins in the blood can damage cerebrovascular endothelial cells and increase BBB permeability. Complement activation may activate glial cells, leading to the release of proinflammatory cytokines and thereby exacerbating BBB damage.25 BBB dysfunction contributes greatly to SAE pathophysiology since the central nervous system is highly susceptible to neurotoxic factors such as free radicals, cytokines, intravascular proteins, and circulating inflammatory cells. Therefore, BBB dysfunction leads to brain edema and reduced microvascular perfusion, exacerbating neuronal loss in SAE.26
SAE-induced BBB dysfunction involves complex cellular and molecular mechanisms. The severity of systemic inflammation during sepsis is intricately linked to the severity of BBB damage and the reduced expression of tight junction proteins.27 The binding of lipopolysaccharide (LPS) to toll-like receptor 4 (TLR4) on endothelial cells triggers the activation of nuclear factor kappa B (NF-κB) via both the MYD88 innate immune signal transduction adapter (MYD88) and FGH1-ras homolog family member A (RHOA) signaling pathways. This activation upregulates genes encoding proinflammatory cytokines and chemokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6, ultimately causing endothelial cell damage and exacerbating BBB impairment.28, 29 The effects of endotoxins and proinflammatory cytokines result in decreased occludin expression, altering endothelial cell integrity and permeability.30 Additionally, during sepsis, mitochondrial dysfunction mediated by the dynamin 1-like (DNM1L/DRP1)-fission mitochondrial 1 (FIS1) pathway, alterations in endothelial cell sphingolipid metabolism, downregulation of tight junction proteins facilitated by matrix metalloproteinases, and detachment of pericytes from the basement membrane all exacerbate BBB damage.13, 24, 31, 32
Damage to the BBB facilitates the entry of inflammatory mediators into the brain, promoting microglia activation. Overactivation of microglia triggers the release of inflammatory mediators, ROS, nitric oxide (NO), and glutamate, aggravating the inflammatory response and exacerbating BBB damage.33, 34 Consequently, the mechanisms of BBB dysfunction in SAE are intricate, necessitating the further exploration of the underlying mechanisms and identification of targeted therapeutic approaches.
2.2 Overactivation of glial cells and subsequent damage
The neurovascular unit is an integral unit composed of neurons, capillaries, microglia, oligodendrocytes, and the extracellular matrix.35 In particular, microglia are defensive cells that respond to various pathogens and neuronal damage and serve as resident and immune-responding cells in the central nervous system. Recent studies have shown that interactions between endothelial cells and microglia are associated with various inflammatory-related brain diseases.36 When the BBB is disrupted, resting microglia are rapidly activated and release TNF-α, IL-6, IL-1β, and other inflammatory cytokines in response to the sepsis-induced inflammatory response.37
The binding of LPS to TLR4 on microglia triggers proinflammatory processes including the production of TNF-α and an increase in glutamate through the junction protein channel and cysteine/glutamate reverse transport system, ultimately promoting the dysregulation of calcium influx and inducing neuronal injury.38 Damaged neurons release the proinflammatory molecule high mobility group box 1 in cooperation with activated microglia and astrocytes through a neuronal TLR4-dependent signaling mechanism to further propagate inflammation.39 In addition, TNF-α and glutamate can mutually enhance their release.40 Microglia activation also induces IL-6 and IL-1β synthesis through the expression of CD40 molecule (CD40) and CD40 ligand (CD40LG/CD40 L) as well as transcription factors such as NF-κB, contributing to the persistence of inflammatory reactions.41, 42
In patients with sepsis and memory impairments, IL-1β released by activated microglia suppresses synapse formation and axon development by activating the p38-mitogen-activated protein kinase (MAPK) signaling pathway.43 In addition, the increase in endothelial cell death and BBB permeability induced by activated microglia is mediated by the Janua kinase (JAK)-signal transducer and activator of transcription (STAT) and c-Jun N-terminal kinase (JNK) signaling pathways. These factors, alone or together with peroxynitrite, have cytotoxic effects on endothelial cells.44 Therefore, suppressing microglial activation can improve the long-term cognitive ability of patients with sepsis.45
2.3 Alterations in neurotransmitters and disturbances in brain signal transmission
In sepsis, inflammatory factors released by the body enter brain tissue through the damaged BBB, causing a stress response, a process regulated by two main pathways. First, inflammatory factors activate axonal cytokine receptors in the vagus nerve, leading to abnormalities in the secretion and regulation of the agonist system due to the connections of the vagal nucleus to various autonomic nuclei in the brainstem, especially the paraventricular nucleus of the hypothalamus, which controls the adrenal axis, the secretion of antidiuretic stimulants, and the nucleus of the solitary tract, which integrates the stress reflex.46 Second, the neuroendocrine and autonomic nuclei near the periventricular apparatus lack the protection of the BBB and are directly acted on by inflammatory factors, causing the release of additional inflammatory factors, anti-inflammatory factors, and components of the innate and adaptive immune systems. These factors (e.g., prostaglandins, chemokines, and NO) can act directly or indirectly on microglia, astrocytes, and neurons, disrupting nerve cell messaging and neuroendocrine secretion and causing signaling disorders in the brain.47, 48
An increase in aromatic amino acids in brain tissue due to a damage-induced change in BBB permeability, an increase in aromatic amino acids in the body, which increases the ratio of aromatic to branched-chain amino acids in brain tissue, and an increase in pseudoneurotransmitter synthesis, which interferes with normal neuronal function, may also be crucial for the pathogenesis of sepsis leading to SAE.49
The cholinergic anti-inflammatory pathway is a recently discovered neuroimmune regulatory mechanism. Studies have shown that SAE is associated with the expression of dopamine, norepinephrine tracts, gamma-aminobutyric acid, 5-light tryptophan receptors, and the cholinergic pathway.50 Animal experiments revealed that the vagal-cholinergic anti-inflammatory pathway may play a protective role in brain- and systemic-damage-related mechanisms by downregulating the expression of inflammatory mediators to alleviate SAE, reduce brain damage, and improve brain functions. Moreover, the cholinergic anti-inflammatory pathway represents a combined therapeutic intervention target for SAE, providing a new approach for preventing and controlling SAE.51 Recently, intraperitoneal injection of the reversible cholinergic enzyme inhibitor methylstaurosporine was shown to be neuroprotective in an animal model of SAE, possibly because methylstaurosporine exerts a certain degree of protection in SAE animals by restoring cholinergic neurologic function and cholinergic anti-inflammatory pathways.52
2.4 Mitochondrial dysfunction and impaired energy metabolism
In the early stages of sepsis, reduced adenosine triphosphate production by mitochondria in response to inflammatory factors, ROS, and NO and deficiencies in energy synthesis may lead to tissue and organ damage.53 Compared to normal brain tissue, more neurons undergo apoptosis during sepsis at specific sites where the BBB is compromised.54 Mitochondrial dysfunction is closely related to brain dysfunction. Sepsis can lead to mitochondrial dysfunction by inhibiting the mitochondrial electron transport chain and uncoupling oxidative phosphorylation (OXPHOS), ultimately resulting in neuronal bioenergetic failure and brain function abnormalities.55
Treatment with BAM15, a mitochondrial uncoupling agent, can alter the cellular energy metabolic state by attenuating the activation of LPS and TNF-α in brain tissues, attenuating mitochondrial damage, and reducing macrophage responses to LPS by shifting their cellular energy state toward anti-inflammatory M2 polarization.56 Mitochondrial transplantation can transform microglia from the proinflammatory M1 to anti-inflammatory M2 type, restore mitochondrial metabolic function and M1-type microglia content, and attenuate neuronal cell death, improving brain functions.57 In addition, SZR-104, a kynurenine acid analog, partially restored OXPHOS associated with complex II but not complex I in the hippocampus, reducing sepsis-associated brain damage.58 Improving mitochondrial energy metabolism can improve SAE symptoms.
3 SAE DIAGNOSIS
There is no specific diagnostic method for SAE. Presently, the SAE diagnosis is mainly based on exclusion; that is, when a patient is confirmed to have an extracranial infection, direct brain infection, metabolic encephalopathy, multiple organ failure, embolism, and adverse drug reactions are excluded, and their clinical symptoms and auxiliary examination findings are combined to make the SAE diagnosis. Currently, the SAE diagnosis relies mainly on each of the criteria in Table 1.59
| Item | Content |
|---|---|
| Symptom | Changes in mental status, particularly alterations in consciousness and cognitive function. |
| Commonly used scales | GCS, MoCA, RASS, CAM-ICU, and APACHE-II. |
| Electroencephalogram | Detecting brain dysfunction by monitoring abnormal signals in brain waves. |
| Imaging studies | Transcranial Doppler ultrasound, MRI, CT, continuous monitoring of bispectral index in EEG, near-infrared spectroscopy, contrast-enhanced ultrasound, dynamic 18F-FDG PET/CT imaging, and functional MRI, among others. |
| Cerebrospinal fluid | SAE lacks specific cerebrospinal fluid biomarkers. Comprehensive cerebrospinal fluid examination is primarily used to differentiate intracranial infections. |
| Biomarkers | NSE and S100β |
- Abbreviations: APACHE-II, acute physiology and chronic health evaluation; CAM-ICU, confusion assessment method for the intensive care unit; CT, computed tomography; EEG, electroencephalogram; GCS, Glasgow coma scale; MoCA, montreal cognitive assessment; MRI, magnetic resonance imaging; NSE, neuron-specific enolase; PET, positron emission tomography; RASS, Richmond agitation-sedation scale; S100β, S100 calcium-binding protein B; SAE, septic-associated encephalopathy.
3.1 Clinical symptoms
In addition to the clinical manifestations associated with sepsis, SAE is characterized mainly by an altered mental status, particularly in consciousness and cognitive function. SAE can occur in the early or late stages of sepsis. The clinical symptoms of SAE in patients in the early stage of sepsis include systemic inflammatory response syndrome and sepsis-related symptoms, as well as mental symptoms such as disorientation, behavioral changes, inattention, and irritability. In patients in the late stages of sepsis, the clinical symptoms of SAE are mainly cognitive impairment, which sometimes manifests as personality changes or depressive states, and these patients are more likely to present with focal and generalized seizures. There are various clinical manifestations of SAE. Diagnosing SAE at an early stage requires a neurological examination to exclude other central nervous system lesions and then using the relevant assessment scales and other auxiliary examination results as reference indices to diagnose SAE, ultimately achieving the goal of early diagnosis and treatment.5, 60
3.2 Common scales
The following scales are commonly used to evaluate the mental status of patients with suspected SAE: the Glasgow Coma Scale (GCS), where a lower score indicates more severe impairment of consciousness; the Montreal Cognitive Assessment, where a score of <26 is highly suspicious for SAE; the Richmond Agitation-Sedation Scale, where a score of >1 or <−1 is significant in diagnosing delirium; and the Confusion Assessment Method for the Intensive Care Unit, which can be used to directly assess mental status in nonsedated patients. In elderly patients, Acute Physiology and Chronic Health Evaluation II scores were significantly greater in those with than without SAE.61-63
3.3 Ancillary examinations
Electroencephalograms (EEGs) are of great value in diagnosing SAE. While one study concluded that 77.46% of patients with SAE will have an abnormal EEG, the specificity is poor.64 Transcranial Doppler ultrasound can be used to assess the blood flow status of patients and can help guide the SAE diagnosis. However, since some patients with SAE have no cerebral circulatory flow changes, transcranial Doppler ultrasound can only be used as an auxiliary diagnostic tool. Magnetic resonance imaging (MRI) and computed tomography (CT) are important but not specific for diagnosing SAE since some patients with SAE have normal MRI and CT findings.65 Continuous EEG dual-frequency index monitoring can be used to assess a patient's state of consciousness. However, studies have shown that its value is significantly lower in patients with SAE and correlates positively with GCS scores.66, 67 An optic nerve sheath diameter of ≥5.5 mm measured using bedside ultrasound effectively diagnosed SAE; however, it had no significant prognostic value.68 Numerous factors affect the optic nerve sheath diameter, and further research is needed.
3.4 Biochemical indicators
The biomarkers neuron-specific enolase 2 (ENO2/NSE) and S100 calcium-binding protein B (S100B/S100β) are widely recognized as key indicators of SAE. NSE and S100β are enriched in neurons and released from damaged neuronal cells into the cerebrospinal fluid in patients with SAE, subsequently entering the bloodstream after the BBB is compromised. In SAE, the N-terminal pro-C-type natriuretic peptide (NT-proCNP) level in peripheral blood increases during nerve injury.69 Neurofilament levels are increased in the plasma and cerebrospinal fluid of patients with SAE and are related to the degree of axonal injury and clinical manifestations.70 Moreover, impaired cholinergic transmission is associated with the onset of delirium, and longitudinal measurements of acetylcholinesterase can help diagnose patients with SAE with delirium symptoms.
Notably, acetylcholinesterase activity changed significantly from baseline for at least five consecutive days in patients with suspected SAE but not in patients with delirium but without SAE.71 This observation indicates that acetylcholinesterase activity could be a significant biomarker for distinguishing SAE from other conditions primarily characterized by delirium.
Additionally, studies suggest that extracellular proteins such as matrix metallopeptidase 8 (MMP8), colony-stimulating factor 3, IL-6, and S100 calcium-binding protein A8 (S100A8) are essential in the pathophysiology of SAE, with S100A8 and MMP8 emerging as potential biomarkers for both its onset and progression.72 Notably, Zhang et al. revealed elevated S100A8 and TNF receptor-associated factor 6 (TRAF6) levels in the peripheral blood of patients with SAE, suggesting a correlation between disease severity and prognosis.73
Additionally, microRNAs, such as miR-370 and miR-155, have been recognized as promising biomarkers for SAE due to their small size, which enables them to cross the BBB and detect diffuse brain dysfunction.74 While these microRNAs generally exhibit high sensitivity, their specificity remains challenging. Despite extensive research on SAE-related biomarkers, it is evident that a single biomarker is insufficient for diagnosing SAE. Various biomarkers may exhibit inconsistent diagnostic and prognostic efficacy for SAE because of their involvement in different stages of its pathophysiological processes. Additionally, the source of specimens for their detection varies. Therefore, a comprehensive approach involving the simultaneous evaluation of multiple biomarkers holds promise for enhancing diagnostic accuracy and efficacy in identifying SAE.
4 SAE TREATMENT
No specific treatment exists for SAE, and treating the primary disease is essential. All patients at risk of sepsis should be screened for SAE through the early detection, diagnosis, and treatment of the underlying sepsis. Notably, the clinical manifestations of SAE may sometimes precede the onset of other sepsis symptoms,1 with unexplained decreases in consciousness and cognitive ability manifesting as early as 36–48 h before the patient becomes symptomatic. However, neurological examination and neuroimaging findings often lack abnormalities. Early screening for delirium, active identification of the source of infection, and timely administration of appropriate treatment can improve the clinical prognosis of affected patients.
Evidence suggests that risk factors for SAE in patients with sepsis include preexisting cognitive deficits, chronic use of psychoactive drugs, comorbid neurological disorders, and altered metabolism (e.g., hypoglycemia, hyperglycemia, hypercapnia, and hypernatremia) during the illness.75 Therefore, maintaining a stable internal environment and actively preventing metabolic disorders is essential. In addition, reducing the use of inappropriate sedative medications, especially benzodiazepines,76 can help reduce the risk of SAE. Recent studies have shown that the α2-adrenergic receptor agonist dexmedetomidine has neuroprotective effects in patients with sepsis by inhibiting neuronal apoptosis and reducing the incidence of impaired consciousness and delirium.77, 78 However, additional studies must confirm its exact mechanism and clinical effects.
It was recently found that treating SAE mice with melatonin improved their survival rate by controlling systemic inflammatory responses and attenuating oxidative stress injury and their abnormal neurobehavior by restoring the expression of hippocampal brain-derived neurotrophic factor, suggesting that melatonin has a potential therapeutic effect on sepsis-associated organ damage and brain dysfunction.79 Recent studies have also shown an altered composition of the intestinal flora in patients with sepsis. Moreover, they demonstrated that introducing functional flora from the feces of healthy individuals into their gastrointestinal tract via fecal microbiota transplantation (FMT) could reestablish a normal intestinal flora composition, thus treating intestinal and extraintestinal diseases. FMT can attenuate microglia activation and reduce the inflammatory response and immune damage to brain tissue by decreasing IL-6, TNF-α, and IL-1β levels in the hippocampus. Therefore, FMT can be used as a therapeutic approach for SAE. Although FMT has not been used in the clinic due to ethical constraints, these findings provide new ideas and methods for clinical and animal research.80-82
Oxidative stress is an important mechanism in SAE. Mitochondrial damage, the inflammatory response, and oxidative stress interact to form a vicious cycle. Therefore, reducing the production of oxidative stress products, such as ROS and reactive nitrogen free radicals, may have some effect in treating SAE.83 Edaravone is an effective antioxidant that can strongly scavenge free radicals and protect nerve cells by preventing lipid peroxidation. In addition, edaravone can inhibit the synthesis of leukotrienes in the brain to protect endothelial cells and reduce brain edema.84 Yin et al. reported that resveratrol glycosides alleviated cognitive dysfunction in LPS-induced SAE by inhibiting endoplasmic reticulum stress in microglia.85 β-Nincotinamide mononucleotide reduced the inflammatory response and oxidative stress in the hippocampus of septic mice via the nicotinamide adenine dinucleotide+/sirtuin 1 pathway, mitigating memory dysfunction and neuronal damage.86 Additionally, antioxidant therapies such as heat shock preconditioning and malvidin have also demonstrated favorable effects in animal experiments.87
While various treatments in animal experiments have shown promising efficacy, clinical research in this area is scarce. Currently, the only registered clinical study is “Adjunctive Sedation With Dexmedetomidine for the Prevention of Severe Inflammation and Septic Encephalopathy (ADVISE) (ClinicalTrails.gov ID: NCT04076826).” This study aimed to assess the impact of two standard sedation protocols (dexmedetomidine sedation vs. propofol/midazolam) on serum biomarkers (S100β) in patients with SAE requiring sedation and mechanical ventilation, providing insights into the therapeutic effects of this approach. Nonetheless, clinical data have yet to be disclosed since the study is ongoing. Therefore, further clinical studies are essential to support the development of SAE treatments.
5 CONCLUSIONS AND FUTURE PERSPECTIVES
Researchers tend to focus on the brain when studying SAE pathogenesis, whereas immunologists often overlook the impact of sepsis on the brain. Animal models of sepsis successfully reproduce the dysfunction of vital organs, such as the heart, brain, liver, lungs, and kidneys, seen in humans. However, conflicting data exist regarding organ failure, neuronal damage, and behavioral changes. To identify new therapeutic targets for SAE and sepsis, further optimization of animal models of SAE is needed, as this will provide a vital foundation for future research on sepsis-related brain injury.
The first step in diagnosing SAE should be to exclude patients with encephalopathy caused by direct infection of the central nervous system, headache caused by head injury, stroke encephalopathy, metabolic encephalopathy, toxic encephalopathy, or drug interference according to their past medical history. In the second step, for patients with suspected SAE, their conscious state should be evaluated with the relevant scoring scales, and then the appropriate, effective, and economical biochemical, neuroelectrophysiological, and imaging examinations should be selected as clinical reference indicators to diagnose SAE. Although various serum markers and electrophysiological methods exist, these ancillary tests lack sufficient specificity and sensitivity. Therefore, rapid, simple, and standardized diagnostic tools for SAE remain a long-term goal. The pathways linking sepsis onset to brain tissue damage and brain dysfunction must be further investigated, and explicitly targeting and blocking these key pathways will likely constitute a new approach to SAE treatment.
AUTHOR CONTRIBUTIONS
Rui Wang: Writing-original draft; writing-review & editing; Visualization. Wanda Bi: Writing-original draft; writing-review & editing. Siyuan Huang: Writing-original draft; writing-review & editing. Qiuju Han: Writing-original draft; writing-review & editing. Jin Deng: Conceptualization; project administration; funding acquisition; writing-review & editing; supervision. Zhen Wang: Conceptualization; project administration; funding acquisition; writing-review & editing; supervision. Ling Zeng: Conceptualization; project administration; funding acquisition; writing-review & editing; supervision. Jianxin Jiang: Conceptualization; project administration; funding acquisition; writing-review & editing; supervision.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (82222038, 82260372 and 81970259), Chongqing Outstanding Youth Fund (CSTB2022NSCQ-JQX0017), High-Level Talent Program Cultivation Project of Army Medical University (2022XRC05), and Guizhou Provincial Science and Technology Plan: SY [2015] 3041. The figures were created by BioRender (biorender.com).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest.
ETHICS STATEMENT
No ethical approval is required as the study does not contain animal or human studies.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




