COVID-19 and cognitive impairment: From evidence to SARS-CoV-2 mechanism
Haodong Pan and Jingyan Niu have contributed equally to the work.
Abstract
Caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), coronavirus disease 2019 (COVID-19) primarily manifests as respiratory dysfunction. However, emerging evidence suggests SARS-CoV-2 can invade the brain, leading to cognitive impairment (CI). It may spread to other brain regions through transsynaptic neurons, including the olfactory, optic, and vagus nerves. Moreover, it may invade the central nervous system through blood transmission or the lymphatic system. This review summarizes the neuroimaging evidence from clinical and imaging studies of COVID-19-associated CIs, including magnetic resonance imaging and 18F-fluorodeoxyglucose positron emission tomography-computed tomography. The mechanisms underlying COVID-19-associated CIs are currently being actively investigated. They include nonimmune effects, such as viral proteins, tissue hypoxia, hypercoagulability, and pathological changes in neuronal cells, and immune effects, such as microglia and astrocyte activation, peripheral immune cell infiltration, blood-brain barrier impairment, cytokine network dysregulation, and intestinal microbiota. Inflammation is the central feature. Both central and systemic inflammation may cause acute and persistent neurological changes, and existing evidence indicates that inflammation underlies the elevated risk of Alzheimer's disease. Finally, potential therapeutic options for COVID-19-associated CIs are discussed. In-depth research into the pathological mechanisms is still needed to help develop new therapies.
Abbreviations
-
- Aβ
-
- amyloid-β
-
- ACE-2
-
- angiotensin-converting enzyme-2
-
- BBB
-
- blood-brain barrier
-
- CCL 11
-
- C-C motif chemokine 11
-
- CSF
-
- cerebrospinal fluid
-
- EEG
-
- electroencephalogram
-
- IFNAR1
-
- interferon alpha/beta receptors 1
-
- IFN-I
-
- interferon I
-
- IL
-
- interleukin
-
- MMSE
-
- Mini-Mental State Examination
-
- MoCA
-
- Montreal Cognitive Assessment
-
- NCD
-
- neurocognitive disorders
-
- NLRP3
-
- NOD-like receptor thermal protein domain associated protein 3
-
- Nsp1
-
- nonstructural protein 1
-
- PCS
-
- post-COVID syndrome
-
- SASP
-
- senescence-associated secretory phenotype
-
- TLR4
-
- Toll-like receptors 4
-
- TMPRSS2
-
- transmembrane serine protease 2
-
- TNF-α
-
- tumor necrosis factor-α
-
- WM
-
- white matter
-
- WMH
-
- white matter hypertension
Key points
What is already known about this topic?
-
Caused by SARS-CoV-2, the effects of coronavirus disease 2019 (COVID-19) are not limited to the respiratory system. It also causes notable neurological and cognitive impairments (CIs), including difficulties in attention, memory, and executive function, driven by a combination of immune and nonimmune mechanisms. Such impairments have far-reaching consequences, impacting individuals' quality of life and productivity in society.
What does this study add?
-
This review comprehensively introduces clinical and neuroimaging studies on CIs associated with COVID-19 and their pathological mechanisms. It also emphasizes the progress, limitations, trends, and potential challenges in clinical and basic research. It focuses on the mechanisms of COVID-19-associated CIs, such as immune inflammation, the blood-brain barrier, neurotransmitters, gut microbiota, and metabolites, proposing possible future research and development directions. It also discusses potential therapeutic approaches for COVID-19-associated CIs, highlighting the importance of further investigating their underlying mechanisms to facilitate the development of new treatments.
1 INTRODUCTION
Since its first outbreak at the end of 2019, the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) remains rampant worldwide, having significant negative effects on the global economy and society. By mid-2022, the global tally of coronavirus disease 2019 (COVID-19) cases had surpassed 540 million, with related deaths exceeding 6 million worldwide.1 From a global economic perspective, the global economy is projected to contract by approximately 8%, disproportionately impacting the economies of poorer countries, and the United Nations expects the global economy to lose about two trillion dollars.2
Besides the severe respiratory symptoms, patients with COVID-19 show neurological deficits, primarily cognitive impairment (CI). During the initial phase, about 34% of individuals affected by COVID-19 experienced impaired memory, concentration, or attention.3 An ongoing prospective cohort study of 226 survivors of COVID-19-related pneumonia indicates a potentially heightened occurrence of CI, with 78% of the participants having poor performance in at least one cognitive domain, with approximately half displaying deficiencies in executive function and psychomotor coordination, ranging between 50% and 57%.4 A recent cross-sectional study used multidimensional immunophenotypic tests and machine learning methods to discover the immunological characteristics and biological features associated with COVID-19, showing significant differences in the circulating myeloid and lymphocyte cell populations, with patients with COVID-19 showing an augmented humoral immune response to SARS-CoV-2. Furthermore, using machine learning, low cortisol levels were highly predictive of long COVID syndrome, which may help develop objective biomarkers for long COVID.5 Notably, even patients with milder SARS-CoV-2 infections showed deficits in attention, working memory, executive function, multitasking ability, and language, collectively termed “brain fog.”6 Therefore, understanding the invasion, clinical, imaging, and pathological effects of SARS-CoV-2 in the brain is vital, and future research must explore the underlying mechanisms that may provide strategies and a theoretical basis for preventing and treating COVID-19-associated CIs after the pandemic.
In addition to its direct neuro-invasion and nonimmune effects, such as severe systemic ischemia-hypoxia, hypercoagulable state, vascular thrombosis, and pathological changes in neuronal cells, one potential main mechanism through which SARS-CoV-2 causes cognitive symptoms could be its effects on the immune system.7 A recent study published in Nature suggests that inflammation is the underlying cause of CI in the brain.8 Inflammation activates microglia, astrocytes, and peripheral immune cells, inducing the abundant release of proinflammatory cytokines, the disruption of the blood-brain barrier (BBB), and changes in the gut microbiota and their related metabolites, which allow inflammation to persist, ultimately causing long-lasting central nervous system (CNS) tissue damage and CI.9, 10
This review summarizes the routes of SARS-CoV-2 entry into the CNS and the clinical and imaging evidence for COVID-19-associated CIs. It also examines the nonimmune and immune mechanisms underlying COVID-19-associated CIs and identifies the challenges and prospects for developing immune-based therapies for COVID-19-associated CIs.
2 ROUTES OF SARS-CoV-2 ENTRY INTO THE CNS
SARS-CoV-2 enters host cells via the binding of its spike (S) glycoprotein to angiotensin-converting enzyme 2 (ACE2) on its surface, which is widely distributed throughout the body, including in the brain. The widespread distribution of ACE2 receptors in the brain suggests that SARS-CoV-2 might directly affect neurological function.11 SARS-CoV-2 may access the CNS through several pathways, including retrograde transport along cranial nerves such as the olfactory, optic, trigeminal, and vagus nerves.12 This retrograde transport could result in neuronal necrosis and dysfunction, contributing to brain injury and CI.13 ACE2 receptors on the surface of cells in the brain may also influence SARS-CoV-2's affinity for neural tissues and its ability to cause neurological damage.14 In particular, studies in macaque monkeys have shown that SARS-CoV-2 primarily invades the CNS through the olfactory bulb,15 which connects directly to the nasal cavity and the CNS.16 This route allows SARS-CoV-2 to bypass the BBB and reach the brain. Additionally, SARS-CoV-2 could reach the CNS through the vagus nerve, which connects various vital organs, including the brain, heart, lungs, and intestines. While human data is limited, rodent studies have shown ACE2 expression in the vagus nerve complex, suggesting a possible pathway for SARS-CoV-2 to spread to the brainstem.17
Concerns have also been raised about the potential for intestinal infection by SARS-CoV-2 since ACE2 receptors are highly abundant in intestinal epithelial glandular cells,18 providing a molecular basis for the susceptibility of the gastrointestinal system to SARS-CoV-2 infection. Additionally, SARS-CoV-2 could reach the brain via the ocular surface through contact with contaminated hands or aerosols. Once on the ocular surface, SARS-CoV-2 may access the brain through the nasolacrimal duct and bloodstream.12
Besides these routes, SARS-CoV-2 may infiltrate the CNS through the hematogenous pathway or lymphatic system.19 Recent research has shown that SARS-CoV-2 can invade cells in the choroid plexus, disrupting the blood-cerebrospinal fluid (CSF) barrier and providing another route for the virus to penetrate the brain.20 While these pathways provide potential routes for SARS-CoV-2 to enter the CNS and cause CI, further research is needed to fully understand the biological relevance and implications of these findings. Additional studies are required to elucidate how SARS-CoV-2 affects neurological function and CI through these various pathways (Figure 1).
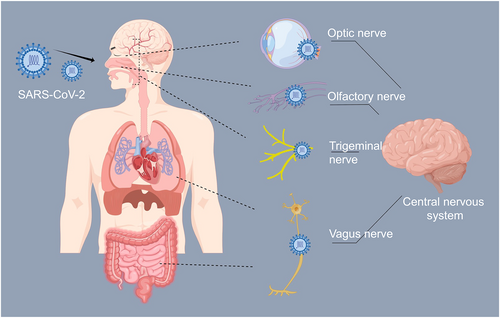
The neuroinvasion pathways of SARS-CoV-2. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
3 CLINICAL AND IMAGING EVIDENCE FOR COVID-19-ASSOCIATED CIs
3.1 Clinical evidence
3.1.1 Clinical presentation
Emerging evidence suggests a significant incidence of CI among patients recovering from COVID-19, with varying prevalence rates across different healthcare settings. In general hospitals, approximately 61.5% of patients with mild to moderate acute COVID-19-related pneumonia develop CI, while in rehabilitation clinics, 12%–80% of patients with moderate to severe symptoms develop CI (Table 1).21 Extended neuropsychological assessments have revealed neurocognitive disorders (NCDs) in around 59.6% of subjects, with 50% showing mild NCDs. Within the cognitive domain, impairments in learning, memory, and executive functions are common, affecting 60.7% of patients with an NCD. Other cognitive deficits include impaired complex attention (51.6%), language (35.5%), and perceptual-motor function (29.0%) (Table 1).22
| Authors (year) | Category | Main findings | Refs |
|---|---|---|---|
| Crivelli L, et al. (2022) | MoCA | Mild to moderate patients: The incidence of CI was 61.5% | [21] |
| Moderate to severe patients: The incidence of CI was 12%–80% | |||
| Schild AK, et al. (2023) | Extended neuropsychological tests | About 59.6% of the subjects had NCDs | [22] |
| Yuan P, et al. (2023) | MMSE | MMSE: Lower attention and calculation scores | [23] |
| MoCA | MoCA: Lower ordinal/inverted digit span, continuous seven-second management, language fluency, and abstraction scores | ||
| Davis HE, et al. (2023) | Retrospective cohort studies | The risks of CI and dementia continued to increase for at least 2 years | [24] |
| Paolini M, et al. (2023) | MRI | Mean diffusivity, radial diffusivity, and axial diffusivity levels in WM were high, while anisotropy values were low | [25] |
| Cecchetti G, et al. (2022) | MRI | MRI: Larger WM hyperintensities volumes | [26] |
| EEG | EEG: Higher regional current density and delta band connectivity and lower EEG delta waves at baseline | ||
| Hugon J, et al. (2022) | FDG PET | Locus coeruleus dysfunction may contribute to cognitive dysfunction | [27] |
| Blazhenets G, et al. (2021) | 18F-FDG-PET/CT | Frontoparietal and, to a lesser extent, temporal hypometabolism were significantly reduced; significant improvements in cognition accompanied this reduction | [28] |
| Ferrucci R, et al. (2023) | 18F-FDG-PET/CT | Significant Aβ deposition in the prefrontal, midfrontal, and posterior cingulate cortexes, with mild extension into the rostral and caudal anterior cingulate cortex | [29] |
| Kas A, et al. (2021) | 18F-FDG-PET/CT | All patients had consistent hypometabolism patterns across a broad brain network, including the frontal cortex, anterior cingulate gyrus, insula, and caudate nucleus | [30] |
- Abbreviations: 18F-FDG-PET/CT, 18F-fluorodeoxyglucose positron emission tomography-computed tomography; CI, cognitive impairment; COVID-19, coronavirus disease 2019; EEG, electroencephalogram; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; MRI, magnetic resonance imaging; NCDs, neurocognitive disorders; WM, white matter.
Specific cognitive deficits have also been observed in patients infected with the Omicron SARS-CoV-2 variant, particularly among those aged >50 years. These deficits include lower scores in attention and calculation tests (e.g., Mini-Mental State Examination [MMSE]) and decreased performance in tasks assessing digit span, continuous seven-second management, language fluency, and abstraction (e.g., Montreal Cognitive Assessment [MoCA]) (Table 1).23 To gain deeper insights into the cognitive impact of COVID-19, large-scale longitudinal studies incorporating affected and unaffected individuals contracting SARS-CoV-2 could be invaluable. Comparing cognitive profiles between these groups may identify the most affected cognitive domains and potentially uncover diagnostic and predictive markers. Additionally, including individuals with pre-existing CI in such studies is crucial for tracking their progress after COVID-19. Further research is warranted to identify the risk factors contributing to the emergence or exacerbation of CI after COVID-19. Early recognition of these factors at symptom onset is essential for prompt intervention and management strategies.
3.1.2 Symptom duration
COVID-19-associated CI appears to mimic the natural cognitive decline typically seen in individuals aged 50–70 years, effectively accelerating brain aging by approximately 20 years. This effect persists and remains detectable even 6 months after infection. Furthermore, studies suggest that mild neurological damage may be evident even in individuals who only experienced mild symptoms. One study involving over 1.3 million patients with COVID-19 indicated that symptoms such as anxiety and depression tended to gradually alleviate over time, while the risk of cognitive dysfunction or even dementia continued to increase for at least 2 years (Table 1).24
CI after COVID-19 is common, and our understanding of this issue is still in its infancy. How long CI can persist and whether it is associated with certain specific clinical characteristics of patients must be further explored. Long-term studies are urgently needed to understand the magnitude and duration of these effects. Screening tools such as the MoCA and MMSE, along with comprehensive neuropsychological assessments, have become crucial in initially detecting CI and tracking its changes and severity. Since patients' cognitive function can be influenced by many factors (e.g., history of hypertension, stroke, or low education), future studies should use stringent exclusion criteria to reduce confounding variables and enhance the robustness of findings. Future understanding of the long-term impact, mechanisms, and treatments will require collaboration among clinicians, researchers, patients, and policymakers.31
3.2 Radiographic evidence
3.2.1 Brain MRI
The first study to investigate associations between brain magnetic resonance imaging (MRI) features and subjective cognitive deficits in survivors of COVID-19 showed profound white matter (WM) microstructure and functional connectivity changes in individuals experiencing new cognitive decline after COVID-19 compared with those without cognitive complaints. Radiologically, increased mean diffusivity and decreased fractional anisotropy (FA) are frequently observed among individuals diagnosed with Alzheimer's disease (AD) and mild CI. Higher radial diffusivity values and lower FA values may reflect areas of disrupted WM integrity. Specifically, that study found widespread mean diffusivity in bilateral WM tracts and radial diffusivity to increase and FA to decrease at the 1-month follow-up (Table 1).25 Overall, 53% of patients were found to have at least one CI domain 2 months after COVID-19 had resolved. Individuals with COVID-19 were found to have larger WM hyperintensity volumes, which were associated with poorer baseline memory function and total cardiovascular risk factors. Lower electroencephalogram delta waves at baseline were suggested to predict poorer cognitive function at follow-up (Table 1).26 Since the integrity of the subcortical WM is essential for maintaining normal cognitive function,32 CI may be one consequence of WM injury in patients with COVID-19. At the 3-month follow-up, it was neuroradiologically confirmed that WM injury and the impaired integrity of brain areas (e.g., the hippocampus) were associated with decreased memory in rehabilitation patients with COVID-19.
3.2.2 18F-fluorodeoxyglucose positron emission tomography-computed tomography
18F-fluorodeoxyglucose positron emission tomography-computed tomography (18F-FDG-PET/CT) revealed neuronal correlates of cognitive decline in patients with COVID-19-related pneumonia, with abnormal findings in two-thirds of patients dominated by frontoparietal hypometabolism. Hypometabolic areas in the pontine region in 18F-FDG-PET/CT scans were found in three patients who experienced cognitive decline after COVID-19, and locus coeruleus dysfunction may contribute to cognitive dysfunction in patients (Table 1).27 In a prospective study assessing eight patients with COVID-19 during the subacute and chronic phases, 18F-FDG-PET/CT revealed a notable decrease in metabolic activity in the frontal and parietal lobes, with a small degree of temporal lobe hypometabolism, accompanied by an evident improvement in cognitive function. In follow-up, the presence of previously established COVID-19-related patterns was significantly reduced and correlated negatively with MoCA performance (Table 1).28
A retrospective study showed that among hospitalized patients with COVID-19, who exhibited the most extensive glucose hypometabolism in the brain, 18F-amyloid PET/CT demonstrated significant amyloid-beta (Aβ) deposition in the prefrontal, midfrontal, and posterior cingulate cortexes, with a mild extension into the rostral and caudal anterior cingulate cortex (Table 1).29 Seven patients with different clinical manifestations of COVID-19-related pneumonia-associated encephalopathy underwent three brain 18F-FDG-PET/CT examinations during the acute phase and one and 6 months after the onset of COVID-19. Although their neurological findings were heterogeneous, they all showed major cognitive and behavioral frontal lobe disorders. All patients had consistently low metabolism across diverse brain networks, including the frontal cortex, anterior cingulate gyrus, insula, and caudate nucleus. Six months after onset, most patients showed improvement in their clinical symptoms. However, they still had varying degrees of CI and persistent abnormalities in 18F-FDG-PET/CT scans in the frontal, insular, and subcortical regions (Table 1).30
It remains to be determined whether neuro-radiographic impairment occurs in patients infected with SARS-CoV-2 variants (e.g., Omicron), whether those experiencing breakthrough infections after vaccination exhibit distinctive radiographic alterations, and whether infection with the SARS-CoV-2 Omicron variant induces unique changes in radiographic images among children. Therefore, investigating and comparing the long-term clinical outcomes of patients in each of the above scenarios would be invaluable.10 Moreover, we must acknowledge the complexity introduced by the possibility of individuals being infected with multiple SARS-CoV-2 variants. Indeed, this complexity complicates isolating and understanding the specific neuroradiographic impacts associated with different SARS-CoV-2 variants, such as Omicron, and cases of breakthrough infections post-vaccination. Future research efforts must strive to address this challenge by using sophisticated analytical methods to differentiate the neuroradiographic changes caused by various SARS-CoV-2 variants. In addition, they must examine potential variations in clinical, psychopathological, and MRI features between individuals who recover normal cognitive status and those who remain impaired during follow-up, which is essential for elucidating markers of individual resilience.
4 PATHOLOGICAL MECHANISMS OF CI AFTER COVID-19
4.1 Nonimmune effects
4.1.1 SARS-CoV-2 structural and nonstructural proteins
SARS-CoV-2 itself may have toxic effects on the brain, and the mechanisms by which it induces CI are currently incompletely understood. The SARS-CoV-2 S protein can induce mitochondrial injury in brain endothelial cells and affect their respiratory function, resulting in variable neurological performance among individuals with COVID-19. The fragment of the SARS-CoV-2 S protein is a toxic amyloidogenic agent, and its co-aggregation with Aβ may exacerbate the pathology of AD, leading to downstream effects such as Aβ amyloidosis in tauopathy, inflammation, and immune responses, triggering long COVID.33 In addition, the S protein itself might also contribute to the pathogenic mechanisms because it triggers a proinflammatory response in endothelial cells in the brain and impairs BBB integrity, which may have further indirect toxic effects on neuronal cells,34 providing a reasonable explanation for neurological complications in individuals with COVID-19 to some extent.
Conversely, the nonstructural protein 1 (Nsp1) of SARS-CoV-2 destroys host cells. However, it was found that expression of Nsp1 but not other SARS-CoV-2 proteins could rescue neuromuscular degeneration and learning/memory phenotypes induced by the 99-aa C-terminal fragment (C99) produced by amyloid beta precursor protein (APP) cleavage by β-secretase. Various mechanisms mediate the protective effects of the Nsp1 protein, including disassembling collided ribosomes, restarting stalled translation, and removing erroneous translation products, providing novel insights into the relationship between neurodegeneration and COVID-19, suggesting that Nsp1 could be used to cure these diseases.35, 36
4.1.2 Ischemia and hypoxia
Ischemic brain injury is an evident pathological process in vascular dementia. Small vessel disease is the predominant contributor to vascular dementia, causing approximately 80% of cases.37 Abnormal WM hyperintensities and microvascular arteriosclerosis associated with small vessel disease can be found in the neuroimaging findings of about 50% of patients with dementia.38, 39 Since SARS-CoV-2 binds to ACE2 receptors to infiltrate the cell, the density of ACE2 receptors in the vascular tissue is reduced after infection. This downregulation negatively affects ACE2 activity and leads to the accumulation of angiotensin II,7, 40 resulting in vasoconstriction, pro-fibrosis, and proinflammatory effects that, in turn, lead to endothelial dysfunction, organ damage, and thrombosis.14
It was reported that 24% of individuals in the convalescent phase after COVID-19 infection experienced a decrease in cerebral tissue oxygen saturation, which was associated with diminished neurological function and overall quality of life.41 Hypoxia-related brain pathology may be a major cause of CI in individuals with COVID-19. Red blood cells are the only carriers of oxygen to the brain tissue to maintain its essential activity. Therefore, thoroughly studying the metabolism and morphology of red blood cells, including identifying potential risk factors and elucidating molecular mechanisms that limit tissue oxygen supply, is recommended to address ischemia and hypoxia after SARS-CoV-2 infection, which will be vital for preventing the CI process caused by varying degrees of hypoxia.42
4.1.3 Hypercoagulable state
Circulating prothrombotic factor levels are elevated in critically severe COVID-19 cases, reflecting a hypercoagulable state.43, 44 Compared with healthy controls, all SARS-CoV-2 cases exhibited significantly elevated levels of fibrinogen and fibrin/fibrinogen degradation products, including D-dimer. Notably, severe cases had higher levels than mild cases.45, 46 Moreover, while inflammatory cytokines can directly influence brain cognitive function, hypercoagulability triggered by inflammation-induced microvascular injury was suggested to be a key determinant of the severity and outcome of COVID-19.47
4.1.4 Pathological changes in neuronal cells
SARS-CoV-2 can still be detected in the brains of individuals with COVID-19 for several months after infection.48 Besides cell death or inflammation, another potential consequence of SARS-CoV-2 infection is neuronal fusion, where neurons fuse with glial cells in mouse and human brain organoids.49 Furthermore, Ca2+ imaging showed that these fusions may severely impair neuronal activity by initiating synchronous discharge or shutting it down altogether, thereby permanently altering neural circuits and function. These effects cause chronic neurological symptoms, providing a new mechanistic insight into how SARS-CoV-2 causes neuropathology and potentially explaining the persistent effects on cognitive function.49
There is no unanimous agreement on the outcomes of SARS-CoV-2 infection of the CNS to date. One study examined the ability of SARS-CoV-2 to invade human brain organoids derived from human stem cells and observed its ability to infect neurons within these organoids using the cellular machinery of neuronal cells for replication. It seems that SARS-CoV-2 enhances the replication of infected cells by augmenting their metabolism, leading to the demise of neighboring uninfected neurons due to decreased oxygen supply.50 In addition, in vitro experiments have demonstrated that SARS-CoV-2 can transit along nanotubes formed between infected cells and neurons. Consequently, healthy neuronal cells in contact with infected cells can become infected. The nanotubes between neurons represent a mechanism that favors immune escape and viral persistence, and they contribute to CI by facilitating the transport of proteins that cause degenerative conditions such as AD.51 Further research is required to explore the factors that predispose patients to CNS infections and identify the order of infection of different cell types within the CNS, which will aid in unraveling the underlying mechanisms of neurological diseases associated with SARS-CoV-2.
4.2 Immune effects
4.2.1 Microglia
Microglia are a crucial subset of endogenous immune cells. Studies have shown that microglia exert dual functions in AD. While the moderate activation of microglia is beneficial for clearing Aβ in the brain, their overstimulation by Aβ or APP can lead to heightened inflammatory responses, potentially exacerbating the neurodegenerative processes in AD.52 Significant microglial activation has been found in the brain of rats with COVID-19-related pneumonia, as evidenced by decreases in the number of microglial branches, nearest neighbor distances, stem areas, regular index and process lengths, and an increase in cell bodies.53 Notably, mild SARS-CoV-2 respiratory infections activated microglia in the subcortical WM of a mouse model, leading to the loss of oligodendrocyte precursors and mature oligodendrocytes, followed by the loss of myelinated axons, thereby impairing neuronal network structure and function.54 Additionally, microglial activation in the hippocampus suppresses neurogenesis, leading to impaired memory formation.54, 55
It has been demonstrated that complement component 1q (C1q)-mediated microglial phagocytosis is the cause of long-term CI induced by the SARS-COV-2 S protein.56 The genes enriched in microglia clusters associated with COVID-19-related pneumonia overlapped with those enriched in AD-associated microglia.57 In addition, several specific genes associated with neuroinflammation (e.g., receptor-interacting serine/threonine kinase 1) were present in COVID-19-related pneumonia-associated microglia. Abundant microglial subpopulations in patients with COVID-19-related pneumonia represent a distinctive microglial state similar but not identical to that previously reported in human neurodegeneration.57 However, the potential precise regulatory mechanisms that mediate the microglia response induced by respiratory infections remain to be elucidated.
Neurodegeneration is associated with microglia-associated inflammatory factors. Tumor necrosis factor-alpha (TNF-α) and interleukin (IL)-1β secreted by proinflammatory microglia impede their endocytosis of pathological Aβ and tau proteins. In contrast, the secretion of IL-2, IL-4, IL-10, and transforming growth factor-beta (TGF-β) by anti-inflammatory microglia and activation of specific receptors, such as triggering receptor expressed on myeloid cells 2, contribute to the repair of learning and memory impairment in AD through multiple signaling pathways and mechanisms.58 Given the highly complex roles of microglia, which can be both neuroprotective and neurotoxic, there is an urgent need to elucidate the crucial regulators that might induce a shift in microglia towards an anti-inflammatory phenotype or modulate the activation triggers of microglia.
4.2.2 Astrocytes
In addition to aberrantly activated microglia, COVID-19 significantly reduced the complexity, length, and total number of astrocyte processes and significantly enlarged astrocyte cell bodies in infected patients.53 COVID-19-associated astrocyte clusters were also identified and characterized by the upregulation of established inflammatory and astrogliosis genes (e.g., interferon-induced transmembrane protein 3 and glial fibrillary acidic protein) and neurotoxic factor chitinase 3 like 1.57 IL-1α, TNF-α, and C1q secreted by activated microglia can induce A1 neurotoxic astrocytes and neurotoxin release, inducing neuronal and oligodendrocyte cell death; astrocytes are indeed promoters of neuronal death in AD.59 In addition, astrocytes were shown to be more likely to participate in Aβ clearance than in production by facilitating Aβ transport across the BBB.59 Therefore, while glial communication is pivotal in the development of COVID-19-associated CI, the mechanism remains unclear. Further studies investigating the critical factors regulating neurotoxic astrocyte activation and their interaction with microglia may be crucial to opening new avenues for relieving or treating CI.
In cultures of human brain cells exposed to SARS-CoV-2, substantial viral infection and replication were observed in astrocytes, with comparatively few infections observed in other cell types, suggesting that astrocytes are a major site of viral infection within the brain.60 Older adults may be more susceptible to COVID-19, potentially due to age-related changes in astrocytes that make them more vulnerable to SARS-CoV-2.61, 62
Astrocytes are essential for neurotransmitter recycling, a critical function in preserving synaptic transmission and neuronal excitability. They play a crucial role in regulating glutamate levels in the brain.63, 64 Recently, de Oliveira et al. found that SARS-CoV-2 infected mixed glial cells had lower L-glutamine levels, and inhibition of L-glutamine decreased viral replication and inflammatory response, impairing neuronal function and affecting neuronal metabolism and synaptic function and plasticity.65 One way astrocytes support neuronal metabolism is exporting lactate, and one significant alteration induced by SARS-CoV-2 infection in astrocytes is reduced lactate levels.65 Therefore, SARS-CoV-2-infected astrocytes show marked metabolic changes, resulting in decreased metabolites used to provide energy to neurons and produce neurotransmitters and the release of one or more neurotoxic factors that remain to be identified,65 leading to increased neuronal death and CI.
4.2.3 Peripheral immune cells
SARS-CoV-2 has been found to elicit immune responses upon entry into respiratory epithelial cells. The proinflammatory immune response is mediated by pathogenic T helper 1 cells and intermediate CD14+ CD16+ monocytes through membrane-bound receptors and downstream signaling pathways, leading to macrophage and neutrophil infiltration into lung tissue, triggering cytokine storms.66 Recent studies investigating the immune cell profiles in the CSF of individuals with neuro-COVID-19 using single-cell sequencing methods67, 68 have identified signs of local immune hyperactivation, extensive clonal T-cell expansion, and weaker interferon (IFN) responses.67 In APP/PS1 mice, respiratory infection increased the infiltration of T cells and natural killer T cells into the brain, leading to enhanced gliosis and Aβ deposition.69 Therefore, activated peripheral immune cells including neutrophils in the cerebral vasculature and brain parenchyma mediate CI via aggravating neuroinflammation, activating central resident immune cells, and inducing the accumulation of brain toxic metabolites.70 The exact mechanism of the inflammatory response of peripheral immune cells, such as T cells, remains to be elucidated. Information gained from studies of other neuroimmune disorders may be helpful in this regard. Furthermore, the immunomodulatory effects of cellular neuroimmune interactions between peripheral and central immune cells still need further elucidation.10
4.2.4 Blood-brain barrier
BBB disruption can lead to the accumulation of toxic components such as fibrinogen in plasma, eventually leading to oligodendrocyte damage and myelin sheath loss.71, 72 In addition, the clearance of brain metabolites, including Aβ, may be impaired, which is associated with the development of cerebral amyloid angiopathy and AD.73, 74 One study observed that 18 of 31 patients with COVID-19 exhibiting neurological manifestations experienced BBB breakdown and leakage (58%), making it the first to observe SARS-CoV-2's ability to induce BBB dysfunction in humans.75 Another study revealed that SARS-CoV-2 infection compromises BBB integrity, which was evident in patients with acute infection and those with long COVID suffering from brain fog.76 This finding indicates that ongoing systemic inflammation and BBB dysfunction are significant features in patients with long COVID experiencing brain fog.76
SARS-COV-2 could induce endothelial cell inflammation and alter BBB integrity via the ACE2 receptor on the human brain microendothelium.77 Therefore, COVID-19-associated CI may be caused by the increased accumulation of toxic components and decreased clearance of brain metabolites. Furthermore, immune cell infiltration through the compromised BBB may contribute to WM injury and CI in patients with COVID-19.78 Nonetheless, BBB involvement in SARS-CoV-2 infection and its role in the neurological consequences of COVID-19 must be explored further. Additional research is essential for unraveling the heterogeneity and signaling mechanisms inherent in the maintenance, destruction, and repair of the BBB.
4.2.5 Inflammatory cytokines
Immunologic events during the acute phase were associated with long-term cognitive sequelae after COVID-19.79, 80 SARS-CoV-2 enters target cells by interacting with the ACE2 receptor, triggering an intricate immune response marked by the activation of various pathogenic immune cells. This activation leads to the release of numerous cytokines and chemokines, including ILs, colony-stimulating factor 2 (CSF2/GM-CSF), C-C motif chemokine ligand 2 (CCL2/MCP1), TNF-α, and TGF-β, culminating in a cytokine storm,81 which has also been detected in patients infected with SARS-CoV-182 and may underlie encephalopathy. Patients with chronic COVID-19 exhibiting cognitive deficits or “brain fog” had higher plasma C-C motif chemokine ligand 11 levels than patients with COVID-19 without CI. Proinflammatory cytokines can increase oxidative stress, damage cell membranes, and downregulate the expression of excitatory amino acid transporters, leading to increased glutamate levels, excitotoxicity, and accelerated neuronal loss.11 Moreover, inflammatory factors in the periphery, including IL-1, IL-6, and TNF-α, compromise BBB permeability by upregulating prostaglandin-endoperoxide synthase 2 (PTGS2/COX2) and activating matrix metalloproteinases. BBB disruption allows these cytokines to penetrate the brain initiating microglial activation and oxidative stress, ultimately leading to synergistic CI.83
Notably, plasma TNF-α levels in the acute phase of SARS-COV-2 infection could predict CI scores.84 Excessively elevated TNF-α levels in COVID-19 correlated negatively with alterations in the functional connectivity of various networks, including the hippocampus, temporal pole, nucleus accumbens, amygdala, and cerebellum.84 The serum TNF-α concentration may underlie prolonged CI in individuals recovering from COVID-19.84 A longitudinal study by Lindbergh et al. showed that TNF-α was associated with gray matter loss, accelerated brain atrophy, and global cognitive decline in older adults.85 Elevated plasma TNF-α levels may be a long-term risk factor for COVID-19-associated CI.85
A senescence-associated secretory phenotype (SASP) similar to that of senescent cells, accompanied by proinflammatory cytokine and chemokine secretion, was observed in patients with COVID-19, indicating that SARS-CoV-2 infection can induce a senescence phenotype, especially in patients with lower respiratory tract infections. If neural stem cells undergo aging after viral infection, they will no longer be able to undergo neurogenesis in areas of the brain that are critical for memory consolidation. The SASP and upregulated peripheral proinflammatory cytokines synergize to cause neuroinflammation, accelerating aging and age-related neurodegenerative conditions.11
4.2.6 Inflammatory pathways
Genome-wide association studies have identified several risk genes within the inflammatory pathway associated with AD,86, 87 potentially offering insights into the pathogenic mechanisms underlying SARS-CoV-2-related CI. The increased levels of IL-1β and IL-18 observed in individuals with COVID-19-related pneumonia suggest the potential activation of the NOD-like receptor protein 3 (NLRP3) inflammasome.88 The SARS-CoV-2 open reading frame 3a (ORF3a) protein can activate the NLRP3 inflammasome.89 Prolonged microglial NLRP3 inflammasome signaling may hinder microglial clearance of Aβ, leading to the pathological accumulation of neurodegeneration-related peptides such as fibrillar Aβ and contributing to CI.90, 91
Toll-like receptor 4 could play a role in recognizing molecular patterns associated with SARS-CoV-2-induced inflammatory responses. Polymorphisms in the TLR4 gene have been associated with changes in host immune responses triggered by the SARS-CoV-2 S protein.89 This association may elevate the risk of long-term CI, especially in genetically susceptible individuals.89 In addition, toll-like receptors 3, 7 (TLR7), and 8 (TLR8) recognize SARS-COV-2 RNA through the type I and type II IFN pathways and induce an inflammatory cascade through nuclear translocation with nuclear factor kappa B (NF-κB).92, 93
IL-6 plays a crucial role in COVID-19 by binding to the IL-6 receptor (IL-6R) and IL-6 cytokine family signal transducer (IL6ST/GP130), activating the Janus kinase (JAK)/signal transducer and activator of transcription 3 pathway.94
Many viruses shed double-stranded RNA after infection, which induces a type I IFN (IFN-I) response in humans. Brain IFN alpha and beta receptor subunit 1 (IFNAR1) has been reported to contribute to the development, progression, and severity of neurodegenerative diseases.95-97 Evidence suggests that aged mice have low IFN-I levels98 and exhibit an exaggerated and stronger IFN-I and proinflammatory response to subsequent viral infection,99 providing a plausible explanation for the vulnerability to acute cognitive deficits. However, the molecular mechanism underlying this selective disruption of cognitive function in aged animals requires further investigation.100 It would be interesting to elucidate the cognitive role of IFN-I at baseline in older adults and patients with CI.100 However, since no direct target or treatment has been identified or developed to regulate neuroinflammation after COVID-19, the above inflammatory pathways reflect potential therapeutic targets for neurodegeneration triggered by neuroinflammation.
4.2.7 Gut microflora
Increasing evidence suggests that gut microbiota dysregulation affects chronic changes in the brain and the subsequent onset of AD.101 Extra-pulmonary disease caused by SARS-COV-2 may also affect the gastrointestinal tract, where the ACE2 receptor is abundantly expressed.102 SARS-CoV-2 infection can cause persistent changes in intestinal mucosal integrity and microbial translocation, increasing NF-κB signaling pathway activity and enhancing the systemic inflammatory response.103 Therefore, SARS-CoV-2 triggers unfavorable interactions with the microbiota through a continuous and circuitous process involving autoantibodies and host cytokine pathways, triggering a series of immune responses. Further research on the effect of SARS-CoV-2 infection on gut bacteria and its metabolites is needed, which could help to identify new disease biomarkers and therapeutic targets for COVID-19-associated CI.
4.2.8 Neurotransmitters
Serotonin is an important neurotransmitter. Decreased serotonin levels can lead to cognitive dysfunction. One recent study has shown that SARS-COV-2 may decrease serotonin through three mechanisms: decreased uptake of the serotonin precursor tryptophan in the gastrointestinal tract, platelet overactivation and thrombocytopenia affecting serotonin storage, and increased turnover of serotonin-metabolizing enzymes.103 In turn, decreased serotonin blocks vagus nerve activity, which impairs hippocampal response and memory. This study established a correlation between the lingering presence of SARS-COV-2, sustained inflammation, increased blood clotting, and disturbances in autonomic function, forming a cohesive pathway. It also provided a plausible rationale for the neurological deficits associated with prolonged SARS-COV-2 presence in individuals experiencing long COVID.104 Reduced serotonin levels were associated with symptom severity, and patients with more severe sequelae often had greater serotonin reductions. In an animal study, administering serotonin precursors or selective serotonin reuptake inhibitors was found to restore serotonin levels and reverse CI, providing a feasible direction for therapeutic intervention.104
4.2.9 Bradykinin
Notably, SARS-CoV-2 increases bradykinin levels in cells, called a “bradykinin storm.”105 Bradykinin and its metabolites exert their proinflammatory and vasoactive effects by activating the G protein-coupled bradykinin receptors B1 (BDKRB1/B1R) and B2 (BDKRB2/B2R).106 B1R expression is also enhanced under inflammatory conditions, leading to the increased release of cytokines through an increase in the bradykinin system, which may explain the cytokine storm observed in COVID-19.107 Prior research has revealed elevated plasma bradykinin levels in patients with AD compared to those without dementia.107 Moreover, patients with AD and severe CI had higher plasma bradykinin levels.107 In patients with AD, CSF bradykinin levels were reduced and correlated negatively with the CSF Aβ40/Aβ42 ratio.107 In addition, bradykinin interacts with the fibrous form of Aβ and co-localizes with Aβ plaques in postmortem human AD brains, suggesting the potential involvement of bradykinin in AD progression.108 B1R is also upregulated in reactive astrocytes located around the hippocampus and Aβ plaques in the brains of AD mice, and blocking B1R protects them from focal brain damage by reducing inflammation and preserving BBB integrity.109 This protective measure also reduced Aβ-induced cognitive decline in AD mice.110 Therefore, it has been hypothesized that SARS-CoV-2 may promote CI by increasing bradykinin levels. Consequently, blocking B1R is an extremely promising treatment strategy in COVID-19-associated CI. Future studies should explore the relationships among COVID-19, bradykinin, and CI.
5 INTERVENTION STRATEGIES
After COVID-19, even when the loss of taste persists for up to one and a half years, it is accompanied by gradual SARS-CoV-2 clearance and restoration of the taste sensation in local taste bud cells, indicating that addressing some persistent symptoms, including CI, after COVID-19 with antiviral therapy is possible.111 In the meantime, neuroprotective therapeutics are needed to prevent the occurrence and reduce the severity of COVID-19-associated CI.
Anti-AD drugs such as aminoadamantane and memantine may be appropriate as future therapies to improve COVID-19-associated CI.112 The development of techniques to aid revascularization and restore erythrocyte metabolism may become integral to new therapeutic strategies. Hyperbaric oxygen therapy can potentially stimulate gene expression and metabolic pathways associated with brain tissue regeneration. It may facilitate neurogenesis and angiogenesis in compromised brain tissue. A case report detailing a patient with post-COVID-19 CI indicated that hyperbaric oxygen therapy could enhance cerebral perfusion and preserve the integrity of the WM brain microarchitecture. Notably, it demonstrated positive effects on microarchitectural alterations in the frontal, parietal, and limbic regions of the brain, leading to improved cognitive performance in affected patients.113
Since neuroinflammation may play a major role in COVID-19 brain injury, anti-neuroinflammatory drugs that can cross the BBB are expected to exert potential therapeutic effects in CI. These therapeutic options may include cytokine antagonists and other pathway modulators such as JAK inhibitors (e.g., baricitinib). Current investigations into anti-IL-6 and anti-IL-1β therapies show promise in limiting long-term cytokine storms and preventing CI.11 Quercetin, ginkgolide, and bilobalide, which may have neuroprotective effects after infection, can improve cognitive complications.114 However, whether the above drugs or methods are truly effective or toxic for treating short-term and long-term COVID-19-associated CI remains to be determined. Therefore, more evidence, especially from extensive clinical trials, is essential to gain a deeper understanding of the therapeutic effectiveness and safety of these agents. In addition, it is reasonable to consider neuroprotective therapeutics to prevent the development and reduce the severity of COVID-19-associated CI. However, since only some patients with COVID-19 develop CI, identifying those who are susceptible is vital, and neuroprotective therapeutics are needed for these patients instead of all patients with COVID-19 to avoid overtreatment.
6 CONCLUSIONS AND FUTURE PERSPECTIVES
The COVID-19 pandemic has led to various CIs, particularly in memory, attention, and executive function.31 Understanding the impact on executive functions, including planning, organization, time management, and adaptability, requires further detailed investigation. Identifying which aspects of executive functions are most affected by COVID-19 and the neurological changes responsible for these impairments is vital. Furthermore, identifying new syndromes caused by COVID-19 and assessing whether they can predict subsequent cognitive decline is crucial since this knowledge is vital for devising effective prevention and treatment strategies.
The brain pathological mechanisms underlying COVID-19-associated CI are incompletely understood and multifaceted. Neuroinflammation may be a crossing point and target, and hypoxia-related brain pathology may be its main trigger. Therefore, the widespread changes observed in the brains of patients with COVID-19-associated CI result from the combined effects of multiple pathogenic factors, both central (e.g., neuroinflammation, BBB disruption, and brain WM injury) and peripheral (Figure 2). Several actions are needed to treat CIs effectively. Firstly, the diverse mechanisms contributing to CI must be thoroughly investigated, including developing imaging techniques to accurately characterize molecular events at the cellular and systemic levels, focusing on brain imaging data from patients with long COVID to reveal the pathological mechanisms within the brain. Secondly, more sensitive cognitive tests are needed to demonstrate how brain damage evolves over time and to identify neuroprotective intervention targets corresponding to distinct mechanisms. Thirdly, optimal combinations of neuroprotective intervention strategies must be determined for patients with diverse lesion severities and varied CI presentations, aiming for precision treatments. Nonetheless, COVID-19 is a relatively new disease, and the pathological or disease processes of the nervous system are relatively slow. Therefore, its impact on the nervous system or cognition requires a longer-term observation, especially the relationship with long COVID.
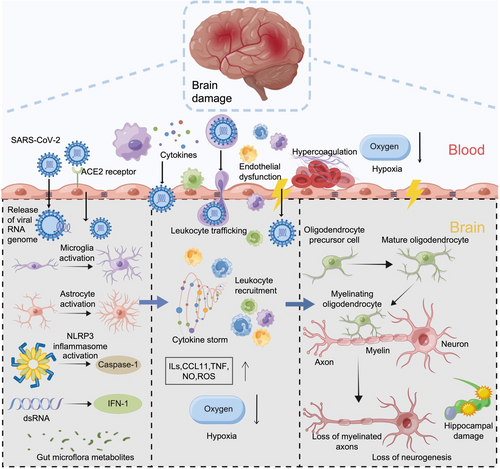
CI mechanisms after COVID-19 at the cellular to molecular level. SARS-CoV-2 may induce CI through diverse mechanisms, including direct toxicity, hypoxia, hypercoagulability, immune-mediated processes involving microglia and astrocytes, BBB dysfunction, and dysregulated cytokine networks. Future research should focus on elucidating regulatory mechanisms governing microglia and astrocyte responses, metabolic alterations in infected astrocytes impacting neuronal function, and the inflammatory role of peripheral immune cells. Additionally, investigating the contributions of the BBB, gut microbiota, and their metabolites to CI and exploring the interplay between COVID-19, bradykinin, and CI are crucial for identifying novel therapeutic targets and treatment strategies for neurological complications of COVID-19. BBB, blood-brain barrier; CI, cognitive impairment; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
AUTHOR CONTRIBUTIONS
Qian Li: Conceptualization; project administration; writing – original draft, writing – review & editing; supervision. Haodong Pan: Writing – original draft; resources. Jingyan Niu: Writing – original draft; resources. Lin Feng: Validation; resources. Yue Yin: Validation; resources. Chun Dang: Validation; resources. Yaoheng Lu: Investigation. Lei Li: Investigation. Jianguang Ji: Writing – review & editing. Kuikun Yang: Writing – review & editing. Lihua Wang: Writing – review & editing.
ACKNOWLEDGMENTS
National Natural Science Foundation of China (82001240), Natural Science Foundation of Heilongjiang Province (YQ2021H011), China Postdoctoral Science Foundation (2020M670925, 2022T150172), Postdoctoral Foundation of Heilongjiang Province (LBH-Z19027, LBH-TZ2019). We also thank Figdraw (www.figdraw.com) for the assistance in creating the Scheme.
CONFLICT OF INTEREST STATEMENT
The authors report no conflicts of interest.
ETHICS STATEMENT
Ethics approval was not needed in this study.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




