Phosphatidylinositol 5-phosphate: A nuclear stress lipid and a tuner of membranes and cytoskeleton dynamics
Abstract
Phosphatidylinositol 5-phosphate (PtdIns5P), the least characterized among the three phosphatidylinositol monophosphates, is emerging as a bioactive lipid involved in the control of several cellular functions. Similar to PtdIns3P, it is present in low amounts in mammalian cells, and can be detected at the plasma membrane and endomembranes as well as in the nucleus. Changes in PtdIns5P levels are observed in mammalian cells following specific stimuli or stresses, and in human diseases. Recently, the contribution of several enzymes such as PIKfyve, myotubularins, and type II PtdInsP-kinases to PtdIns5P metabolism has gained a strong experimental support. Here, we provide a picture emerging from recent studies showing how this lipid can be generated and act as a regulator of membrane and cytoskeleton dynamics, and as a modulator of gene expression. We briefly summarize the current methods and tools for studying PtdIns5P, and discuss how PtdIns5P can integrate and coordinate different functions in a spatiotemporal manner.
Abbreviations
-
- EGFR
-
- epidermal growth factor receptor
-
- IpgD
-
- invasion plasmid gene D
-
- MTM
-
- myotubularin
-
- PHD
-
- plant homeo domain
-
- PI5P4K
-
- phosphatidylinositol 5-phosphate 4-kinase
-
- PtdIns
-
- phosphatidylinositol
-
- PtdIns5P
-
- phosphatidylinositol 5-phosphate
Introduction
Phosphatidylinositol 5-phosphate (PtdIns5P) was described for the first time in mammalian cells in 1997 by Rameh et al. 1. It is the least characterized phosphatidylinositol monophosphate and its role as a bioactive lipid is just emerging. Recently, this lipid was found in unicellular eukaryotes, plants, worms, flies and fish 2-6, suggesting, as for PtdIns3P, an early appearance in eukaryotic evolution. While PtdIns4P is by far the most abundant isomer of phosphatidylinositol monophosphates (representing more than 70%), PtdIns3P and PtdIns5P are present in relatively low amount in mammalian cells (commonly 10–15% of total phosphatidylinositol monophosphates each with considerable variation according to the type of tissue considered). Although our knowledge about PtdIns5P is still rudimentary, it is becoming obvious that similarly to PtdIns(3)P 7 and PtdIns(4)P 8, it is not simply an intermediate metabolite of synthesis of phosphatidylinositol bisphosphates or trisphosphates. In recent years, several striking findings have provided clues to PtdIns5P functions, and a series of groundbreaking discoveries has stimulated the interest of scientists working in this research field. Among those, the characterization of type II PtdInsP-kinases as major enzymes driving the turnover of PtdIns5P was reported 1, 9, providing a tool to establish a method to quantify this lipid 10. Since then, important stepping-stones have marked the research on PtdIns5P: (i) the demonstration that extra-cellular stimuli or stresses induce changes in PtdIns5P levels 10-13; (ii) the discovery of a nuclear protein as the first binder (through its plant homeodomain, PHD) of a nuclear PtdIns5P pool identified this lipid as a nuclear transducer of stress signaling 11, 14; (iii) evidence that PtdIns5P can be produced from PtdIns via PIKfyve 15 or from PtdIns(3,5)P2 via myotubularins 16, 17; and (iv) that a pathogen responsible for bacillary dysentery in humans injects a phosphatase to hijack the host cell signaling and trafficking via transformation of PtdIns(4,5)P2 into PtdIns5P. This last observation suggested that PtdIns5P has important cytosolic functions in determining cell fate 18. As shown for other phosphoinositides, it is increasingly evident that several pools of PtdIns5P exist in membranes facing the cytosol 19, and in the nucleus, in order to control and integrate major cellular processes 20. Herein, we give an overview of the contribution of different research groups whose findings provide the foundation of our knowledge on this rising star of the phosphoinositide family.
PtdIns5P metabolism: Kinases and phosphatases
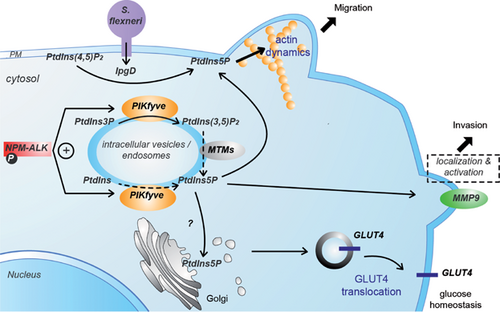
Three potential pathways have been proposed to generate PtdIns5P in mammalian cells (Fig. 1).
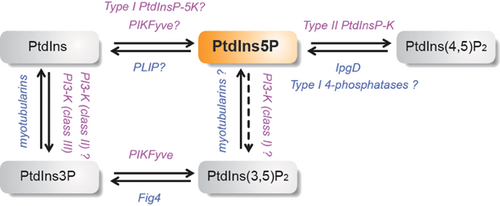
Generation of PtdIns5P by phosphorylation of PtdIns
In vitro, two types of PtdInsP 5-kinases are able to produce PtdIns5P from PtdIns, namely the type III PtdInsP 5-kinase PIKfyve 15 and type I PtdIns4P 5-kinases 21. In vivo, however, data remain controversial. While there are no data available concerning the implication of type I PtdIns4P 5-kinases, the contribution of PIKfyve – the ortholog of the yeast kinase Fab1p initially described to produce PtdIns(3,5)P2 by phosphorylation of PtdIns3P 22 – has been suggested in several studies (see 23 for a recent review). PIKfyve is ubiquitous, and its structure is highly conserved from yeast to multicellular eukaryotes. This kinase, which harbors a Zn2+/PtdIns3P binding domain (called FYVE domain) 24, 25, partitions to localize late endosomes, and possibly to EEA1-bearing early endosomes 26. Several studies have implicated PIKfyve in vesicular transport through the regulation of PtdIns3P/PtdIns(3,5)P2 balance in endosomal membranes 27. Mutations in human PIKfyve are found in a rare autosomal dominant corneal dystrophy, François-Neetens Mouchetée Fleck Corneal Dystrophy 28, and its knockout in mouse is lethal in the embryo, while heterozygous mice are normal 29.
Fibroblasts isolated from heterozygous PIKfyveWT/KO mice have reduced levels of PtdIns5P 29, as do cells treated with a pharmacological inhibitor of the kinase 30, 31, cells treated with siRNA 25 and cells overexpressing a dominant negative mutant of PIKfyve 32. Conversely, over expression of wild type PIKfyve increases its amount 15. The implication of PIKfyve in PtdIns5P production is strengthened by the study of a hypomorph PIKfyve mice model – where a 50% decrease of PtdIns5P is observed 17 – and by a very recent report showing that PtdIns5P is increased upon viral infection via PIKfyve 33.
However, a clear demonstration that PIKfyve produces PtdIns5P by direct phosphorylation of PtdIns in vivo is problematic. Indeed, PIKfyve may, in fact, indirectly control PtdIns5P level by producing PtdIns(3,5)P2, which is then transformed into PtdIns5P by the action of 3-phosphatases of the myotubularin family 17, 34.
Generation of PtdIns5P by dephosphorylation of PtdIns(3,5)P2
Myotubularins (MTMs) are a large family of 3-phosphatases with a substrate specificity restricted to PtdIns3P and PtdIns(3,5)P2 16, 35. About one third of the myotubularin family members are inactive and regulate the activity and/or localization of the active phosphatases through dimerization 36. Several reports have shown that myotubularins can transform PtdIns(3,5)P2 into PtdIns5P in vitro and likely in vivo 16, 35, 37, 38. It has been shown that PtdIns5P can be an allosteric activator of MTM1 and MTMR3, and a component of a positive feedback loop for myotubularin activity 37 – possibly through binding to the PH-GRAM domain 39.
MTM1 is mutated in the centronuclear myopathy, a severe genetic disease linked to the X chromosome, leading to a defect in myotube maturation 40. MTMR2 and the inactive member MTMR13 are mutated in two forms of Charcot-Marie-Tooth Disease (CMT4B1 and CMT4B2), a neuropathy involving defective myelination and myelin out folding 41, 42.
Several studies in mammalian cells, yeast and C. elegans have shown that myotubularins localize to the endosomal compartment, hence their putative role as negative regulators of the endocytic trafficking 43-47. The pathway involving PtdIns3P/PIKfyve/PtdIns(3,5)P2/Myotubularins to produce PtdIns5P – well described in vitro – has recently been suggested to be operative in different in vivo models 17, 34, a concept that is strengthened by phylogenetic analysis 48. This production of PtdIns5P is likely localized to internal membranes where PtdIns(3,5)P2 is located. However, an indirect implication of myotubularins in PtdIns5P production remains possible (see Box 1), and further work is needed to demonstrate whether the transformation of PtdIns(3,5)P2 into PtdIns5P corresponds to a steady-state level of this lipid or whether this pathway can be modulated by regulatory inputs.
Box 1.
PtdIns5P in yeast: Where does it come from?
Is there PtdIns5P in yeast?
Early studies did not report the presence of PtdIns5P in yeast 22, 49-51, and type II PtdInsP-kinases are not found in yeast.
More recently, three studies, using two different techniques, reported the presence of PtdIns5P in yeast in different conditions: (i) a low, albeit detectable, level of PtdIns5P was measured in the Δymr strain (Ymr is the only myotubularin in yeast) by a mass assay 2; (ii) in the ΔFig4 strain, a level of PtdIns5P accounting for about 0.1% of the PtdIns was measured by HPLC after a metabolic labeling with [3H]-inositol 52, and (iii) PtdIns5P was detected in a wild type strain by HPLC after metabolic labeling with [3H]-inositol, and its level was reduced by 1.7-fold in the ΔFab1 strain 17.
Which metabolic route to the production of PtdIns5P in yeast?
- According to Parrish et al. 51, Ymr controls PtdIns3P levels in conjunction with Sjl3p but does not use PtdIns(3,5)P2 in vivo, which is an argument against the production of a constitutive PtdIns5P pool from PtdIns(3,5)P2 by the yeast myotubularin.
- PtdIns(3,5)P2 is the least abundant phosphoinositide in yeast and is barely or not detectable by HPLC after metabolic labeling under basal conditions 2, 22, 49, 51, 52, suggesting that there is not enough PtdIns(3,5)P2 to produce PtdIns5P. One could argue that the way in which PtdIns(3,5)P2 and PtdIns5P are measured is misleading: PtdIns(3,5)P2 is measured by HPLC after a metabolic labeling (particularly, 32Pi labeling may ignore a potential poorly metabolic pool of PtdIns(3,5)P2) and PtdIns5P is measured by a mass assay, making it difficult to compare the levels of phosphoinositide measured with these two different methods.
- In the ΔFig4 strains, ectopic expression of rat MTMR2 induces a 2.5-fold increase in PtdIns5P 52. In the Δymr strain, expression of human MTM1 increases PtdIns5P by 10-fold above basal, which is not observed with the hMTM1 phosphatase dead mutant MTM1C375S 52. These observations are in favor of (i) a phosphatase activity by MTM1 and MTMR2 leading to this increase in PtdIns5P and (ii) the presence of PtdIns(3,5)P2 in sufficient quantity to produce PtdIns5P in those conditions. Another possibility is that hMTMR1 could, as shown for Fig4, activate PIKfyve, leading to an increase of PtdIns5P via PtdIns phosphorylation. Overexpression of MTM1 could hijack PIKfyve from the PAS (PIKfyve-ArPIKfyve-Sac3) complex.
- Ectopic expression of PIKfyve in a yeast WT strain increases PtdIns(3,5)P2 by fivefold but only weakly PtdIns5P, suggesting that PtdIns5P may come from PtdIns(3,5)P2 dephosphorylation 17.
- Under osmotic shock there is a strong increase of PtdIns(3,5)P2, which could then serve as a reservoir for PtdIns5P production 52. Under this condition, when rMTMR2 is expressed there is a transient decrease in PtdIns(3,5)P2 and an increase in PtdIns5P, suggesting that, in these conditions, PtdIns(3,5)P2 could be transformed into PtdIns5P via myotubularins.
In conclusion, these data suggest that PtdIns5P can be produced in yeast, but the question of its origin is not clearly answered. The discrepancies observed suggest that “PIKfyve” and “PIKfyve/myotubularin” routes may contribute to constitutive or inducible PtdIns5P, one or the other being favored depending on the physiological conditions. Further work is needed to clarify this point and to demonstrate the direct or indirect implication of these enzymes.
Generation of PtdIns5P by dephosphorylation of PtdIns(4,5)P2
This pathway was first described in mammalian cells infected by the bacterial pathogen Shigella flexneri 18. This pathogen uses a type III secretion system to inject virulence factors into the host cell to promote its uptake 53. The virulence factor IpgD (Invasion plasmid gene D) has a motif related to the mammalian inositol 4-phosphatase active site (CX5R = CKSGKDRT), and is able to transform PtdIns(4,5)P2 into PtdIns5P both in vitro and in vivo 18. During infection with S. flexneri, there is a significant hydrolysis of PtdIns(4,5)P2 and a concomitant accumulation of PtdIns5P initially in the plasma membrane at the bacterial entry site (Fig. 2) 18. IpgD has a marked preference for PtdIns(4,5)P2 as a substrate over the other phosphoinositides.
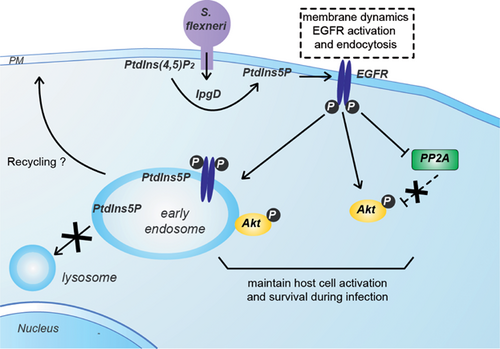
Two homologs of IpgD, SopB in Salmonella dublin and SigD in Salmonella typhimurium (responsible for gastroenteritis) also have motifs related to the mammalian inositol 4-phosphatase active site 54. These enzymes can hydrolyze diverse phosphoinositides and inositol phosphates 55. It has been shown that Salmonella infection induces PtdIns5P production in a SigD-dependent manner, indicating that, similarly to IpgD, these enzymes can transform PtdIns(4,5)P2 into PtdIns5P during the early phases of infection 56. The model of S. flexneri infection and IpgD-mediated PtdIns5P production is useful for investigating the role of this lipid in a pathological situation where an infectious agent hijacks the signaling machinery of host cells to establish its virulence (Fig. 2). A database search based on the conserved CX5R phosphatase motif led to the discovery of two mammalian phosphatases, namely type I and II PtdIns(4,5)P2 4-phosphatases, which are both able to convert PdtIns(4,5)P2 into PtdIns5P 57, 58. It remains to be established whether they can transform PdtIns(4,5)P2 into PtdIns5P in physiological or pathological situations. Both enzymes are ubiquitously expressed and colocalize with EEA1 and LAMP1, two endosomal/lysosomal specific markers. Again, this finding is consistent with a role of PtdIns5P in the control of vesicular trafficking in the endo-lysosomal compartment.
Removal of PtdIns5P by type II PtdInsP-kinases
According to our current knowledge, there is only one major intracellular pathway for removing PtdIns5P. This route involves type II PtdInsP-kinases (also called PtdIns5P 4-kinases), which use PtdIns5P as a substrate to convert it into PtdIns(4,5)P2 1. In 1997, it was shown that although structurally homologous to the type I PtdInsP-kinases (also called PtdIns4P 5-kinases) – which use PtdIns4P as a substrate – type II PtdInsP-kinases phosphorylate the 4th hydroxyl group of the inositol ring of PtdIns5P to generate PtdIns(4,5)P2 1. These enzymes are conserved throughout evolution, from fly to zebrafish, worms and humans 3. Mammalian genomes contain three genes that encode three isoforms of type II PtdInsP-kinases (α, β, and γ) 3. Type II PtdInsP-kinase β and γ are much less efficient than the α isoform at transforming PtdIns5P into PtdIns(4,5)P2 13. Because of its high substrate selectivity and specific activity, recombinant type II PtdInsP-kinase α is now used to monitor the amount of PtdIns5P in cell extracts via a specific mass assay 10. The increase in PtdIns5P levels in the nucleus of mammalian cells following oxidative stress by hydrogen peroxide or UV irradiation has been shown to be due to inhibition of type II PtdInsP-kinase α and β 4, 11. Moreover, dPIP4K, the ortholog of type II PtdInsP kinase in Drosophila controls PtdIns5P levels in vivo and regulates cell growth during larval development 5.
In normal cells, the amount of PtdIns(4,5)P2 synthesized from PtdIns5P is minor compared to the amount formed by the canonical PtdIns4P 5-kinases pathway, hence it is currently accepted that type II PtdInsP kinases are more efficient in controlling PtdIns5P levels than in producing significant amounts of PtdIns(4,5)P2. However, a very localized production of PtdIns(4,5)P2 from a specific pool of PtdIns5P cannot be ruled out 19. There is clearly much to learn about the coordinated functions of these kinases in the course of furthering our insight into the function of localized pools of PtdIns5P.
Other pathways of PtdIns5P degradation might still have to be characterized. For instance, a PTEN-like phosphatase from Dictyostelium, PLIP, has been shown to preferentially hydrolyze PtdIns5P in vitro 59. The capacity of PLIP to dephosphorylate PtdIns5P in vivo has, however, not been demonstrated, and several other roles have been assigned to this phosphatase. Finally, it is also plausible that a putative catabolic pathway involving hydrolysis of PtdIns5P by phospholipases 13 exists: this pathway would involve phospholipase Cδ, and would generate inositol 1,5 bisphosphate 60.
Current approaches to quantifying and localizing PtdIns5P
Quantification of PtdIns5P
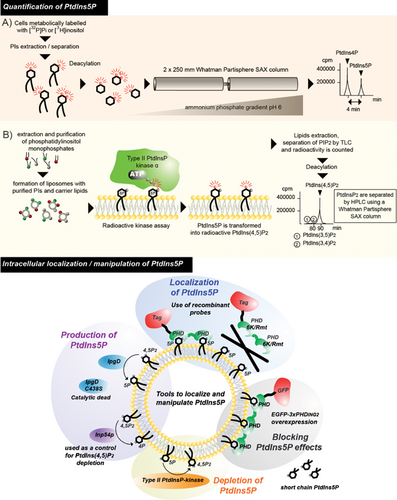
Two different approaches have been described for quantifying PtdIns5P, and are illustrated in Fig. 3. The first method allows the distinction between PtdIns5P and PtdIns4P, which is much more abundant, using anion-exchange HPLC (Fig. 3A). The second method is based on a specific PtdIns5P mass assay using recombinant type II PtdInsP-kinase α, which phosphorylates PtdIns5P into PtdIns(4,5)P2 (Fig. 3B). Using these methods, increases in PtdIns5P could be demonstrated in a number of models and conditions (see Table 1) and was also detected in fish 65 and flies 5. Its presence in yeasts remains a point of discussion as highlighted in Box 1. Mass spectrometry methods have been recently developed to quantify phosphoinositides. However, the difficulties to efficiently distinguish the isomers of phosphatidylinositol monophosphates make the development of this appealing approach very challenging for PtdIns5P quantification.
| Stimulus | Models | References |
|---|---|---|
| Thrombin | Human blood platelets | 10 |
| TCR | T cells | 61 |
| Oncogenic stimulation | Lymphoma cells | 62 |
| Insulin | 3T3-L1 adipocytes | 12 |
| Osmotic-stress | 3T3-L1 fibroblasts, 3T3-L1 adipocytes, and insect Sf9 cells | 63 |
| H2O2 treatment | U2OS; MDA-MB-468; HT1080; p53-null murine embryonic fibroblasts; HeLa | 4, 64 |
| UV irradiation | MEL | 11 |
| Infection with S. flexneri or Salmonella enterica | HeLa | 18, 56 |
Localization and manipulation of PtdIns5P pools
When and where PtdIns5P is produced and metabolized within cells remain largely unanswered questions. A biochemical subcellular fractionation of resting [3H]-inositol-labeled cells followed by HPLC quantification of PtdIns5P has indicated that the majority of the basal PtdIns5P is present in the plasma membrane and intracellular membrane compartments, including smooth endoplasmic reticulum and/or Golgi 19. Using the mass assay and a different subcellular fractionation method, PtdIns5P was found enriched in early endosomes of cells expressing IpgD 66. Moreover, it has been shown that isolated nuclei contain PtdIns5P, the concentration of which increases as cells progress through the cell cycle 67 and upon stress 4, 6, 11, 58. Despite some limitations due to potential relocation or degradation of the lipids during the isolation procedures, these data strongly suggest the existence of distinct pools of PtdIns5P. For imaging purposes, it is possible to localize this lipid by using the first PtdIns5P binding domain described, namely the plant homeodomain of ING2 14. Tools for manipulating PtdIns5P levels experimentally, and to disrupt its functions in cells, are illustrated in Fig. 3. However, imaging PtdIns5P in resting normal cells containing low concentrations of this lipid is challenging, because of the relatively weak avidity of this PHD domain for PtdIns5P. A series of PtdIns5P biosensors showing high avidity for this lipid would be a welcome tool for investigating the distribution of PtdIns5P under physiological conditions.
PtdIns5P in the plasma membrane and intracellular membranes
A role in signaling and vesicular trafficking
As mentioned above, increases in PtdIns5P can be detected in response to various stimuli, and a role for this lipid in signal transduction has been demonstrated on several occasions. Indeed, three independent studies have shown a link between PtdIns5P production and Akt/PKB activation. During the early phase of S. flexneri infection, PtdIns5P produced by IpgD at the plasma membrane induces activation of the Akt pathway, a mechanism whereby the pathogen promotes survival of its host cell (Fig. 2) 68. Consistent with this finding, overexpression of type II PtdInsP-kinase β has been shown to attenuate insulin-induced Akt activation 69, while deletion of type II PtdInsP-kinase β in mice increases insulin sensitivity by enhancing Akt activation in muscle 70. Moreover, in T cells, PtdIns5P is rapidly produced upon TCR activation, and regulates Dok adaptator proteins phosphorylation and recruitment by binding to their PH domain 61, 71. This suggests a role of the lipid in regulating the stoichiometry of signaling proteins at the plasma membrane during lymphocyte activation (see 72 for a review). Elevation of PtdIns5P has been reported in human blood platelets (which lack a nucleus) following thrombin stimulation 10. This increase is independent of platelet aggregation (Payrastre, personal observation), but its role in the regulation of platelet function is still unknown.
In the case of S. flexneri infection, the initial pool of PtdIns5P produced by IpgD is located at the entry foci of the plasma membrane; here it induces intense membrane dynamics together with a modulation of hemichannel opening 73 and an activation of the epidermal growth factor receptor (EGFR) independently of its ligand 66. Interestingly, this EGFR activation is required for PtdIns5P-dependent activation of Akt. The activation of this survival pathway is prolonged due to an important modification of the trafficking machinery. Indeed, PtdIns5P specifically alters the degradative pathway (trafficking of cargoes such as receptors from endosomes to lysosomes), whereas it spares the recycling pathway (endosomes to plasma membrane) and the retrograde pathway (endosomes to Golgi). PtdIns5P was found enriched in endosomes 66, where it maintains activation of EGFR and its downstream effectors such as Akt at the early endosome compartment. PtdIns5P has a profound inhibitory effect on EGFR degradation. However, the molecular mechanisms used by PtdIns5P to activate EGFR and modulate its degradation are still unknown. In relation to the latter point, it could be hypothesized that the increase in PtdIns5P at endosomes could recruit effectors and/or alternatively modify the balance of PtdIns5P/PtdIns3P, resulting in a modification of the sorting of the EGFR. Its effect on Akt activation also results from the inhibitory effect of the EGFR on the phosphatase PP2A, a negative regulator of Akt 74. Thus, the model of S. flexneri infection indicates a role for PtdIns5P in coordinating membrane dynamics, signaling and trafficking. In this context, it is noteworthy that endosomes are emerging as central spatiotemporal organizers of signaling circuitry 75.
PtdIns5P, a new player in regulating vesicular trafficking besides PtdIns3P and PtdInds(3,5)P2?
A phylogenic study has shown that PtdIns5P metabolizing enzymes in eukaryotes, including PIKfyve, myotubularins and type II PtdInsP-kinases, are evolutionary conserved as regulators of endosome functions 48. Other indirect evidence also suggests a role for PtdIns5P in vesicular trafficking. PIKfyve and myotubularins likely work in concert to produce PtdIns5P 17, 34, but they also regulate the level of PtdIns3P and PtdIns(3,5)P2, the role of which in vesicular trafficking is now recognized from yeast to multicellular eukaryotes. To better understand the complex roles of these enzymes, PtdIns5P has to be included in the picture. PIKfyve partly localizes to PtdIns3P enriched endosomes through binding to its FYVE domain 32, and various studies implicate the kinase in trafficking and autophagy in mammalian cells (for a review, see 25). PIKfyve inhibitors, siRNA-mediated silencing or overexpression of a dominant negative PIKfyve kinase mutant induce pronounced formation of enlarged, “swollen”, vesicles of endosomal nature 76, 77. It is still unclear whether these swollen vesicles impair normal trafficking of receptors or just the fluid phase endocytosis 78. This role of PIKfyve was first attributed to a defect in PtdIns(3,5)P2 production, acting on the endolysosomal compartment. However, the role of PtdIns5P production in this process was not questioned at the time, and has now to be integrated into the model.
Another report shows a PtdIns5P pool controlled by PIKfyve in intravesicular membranes (using the 2xPHDING2 probe) in lymphoma cells 79. In this model, PIKfyve is required to localize the metalloproteinase MMP9 at the plasma membrane, where it is complexed with CD44 and the chaperone Hsp90, which activates it to control cell invasion 62.
Similarly to PIKfyve, myotubularins play a role in vesicular trafficking and autophagy. While these phosphatases control PtdIns3P, PtdIns(3,5)P2 and PtdIns5P pools, until now their roles were mostly attributed to effects on PtdIns3P or PtdIns(3,5)P2. MTMR4 localizes to early and recycling endosomes and regulates trafficking of the transferrin receptor and indirectly TGF-β signaling 80. MTM1 localizes to Rab5-positive endosomes, while MTMR2 is on Rab7-positive endosomes, and both enzymes may play a sequential role in the control of PtdIns3P pools as well as EGFR sorting and degradation 43. Reminiscent of the effect of IpgD-induced PtdIns5P production 66, MTMR2 overexpression induces a sustained Akt activation and inhibition of EGFR degradation 81. These data suggest that MTMR2 could also be involved in regulating endosomal PtdIns5P together with PIKfyve and/or endocytosis of a PtdIns5P pool produced at the plasma membrane. Future work will help answering this important question.
Altogether, these data are compatible with a model in which a balance between PtdIns3P, PtdIns(3,5)P2, and PtdIns5P regulates discrete steps of endosomes to lysosomes trafficking. Aberrant production of one of these lipids (which are normally produced in a highly controlled spatiotemporal manner) could therefore affect the process. It can be speculated that the ratio between these phosphoinositides in specific subcompartments is vital for ensuring the proper routing of cargoes through the endosomal network – sorting them for recycling or degradation – through the recruitment of specific protein complexes and adaptors. The ratio PtdIns5P/PtdIns(3,5)P2, potentially regulated by a protein complex comprising PIKfyve and myotubularins 82, may be a key factor in ensuring proper targeting of receptors to the degradative pathway, a mechanism exploited by pathogens and deregulated in diseases.
PtdIns5P in cytoskeleton dynamics
Several studies in different models suggest that PtdIns5P can impact on actin reorganization. A preliminary observation indicated that infection of HeLa cells by S. flexneri induces a loss of F-actin stress fibers and a strong actin remodeling at the site of bacterial entry 18. However, it was difficult to demonstrate that this effect was due to PtdIns5P, since IpgD decreases PtdIns(4,5)P2 levels and S. flexneri also injects other factors acting on the cytoskeleton. More conclusive are the observations that ectopic expression of IpgD induces a disappearance of F-actin stress fibers and membrane blebbing reminiscent to what is observed upon activation of small GTPases of the Rho family, Cdc42, and Rac1, respectively, when cells are challenged with bradikinin or EGF 18. The effects on F-actin stress fibers loss were also observed following addition of exogenous PtdIns5P but not by PtdIns3P or PtdIns4P (J. Viaud & F. Gaits-Iacovoni, personal observations). More insights came from the study of insulin stimulation in 3T3-L1 adipocytes, which respond by inducing actin dynamics characterized by disassembly of F-actin. In this model, insulin induces PtdIns5P production in a PIKfyve-dependent manner 12, 15, 30. While microinjection of PtdIns5P into cells reproduced actin stress fibers dynamics observed following insulin treatment, expression of the PtdIns5P-trapping probe (EGFP-3xPHDING2) blocked this effect, suggesting a direct role of PtdIns5P in this process 12. Interestingly, this PtdIns5P-dependent F-actin breakdown was associated with translocation of vesicles containing the GLUT4 glucose transporter (involved in glucose homeostasis) from intracellular membrane pools to the plasma membrane. The involvement of F-actin in the insulin-dependent trafficking of GLUT4 vesicles has been extensively documented 83-85. The effectors linking PtdIns5P to actin cytoskeleton dynamics remain to be identified, but clues point to Rho GTPases pathway components as putative effectors. In insulin-stimulated mature adipocytes that do not have stress fibers, the small GTPase TC10 has been shown to be instrumental in the remodeling of a pool of F-actin localized around the Golgi and required to allow transport of GLUT4 vesicles to the plasma membrane 86. In addition to the plasma membrane, pools of PtdIns5P have been found on intracellular vesicles such as SER/Golgi-derived vesicles involved in transport towards the ER, plasma membrane or late endosomes 19. It is therefore conceivable that PtdIns5P could regulate actin dynamics to power vesicle trafficking following these pathways.
In line with these observations, recent studies have identified PtdIns5P as the central molecule regulating cell migration in response to FGF1 in fibroblasts and border cells during Drosophila embryogenesis 34, 87. These studies revealed that PIKfyve and MTMR3 act together to produce PtdIns(3,5)P2 and its metabolite PtdIns5P. Through rescue experiments with either synthetic lipid or expression of IpgD, PtdIns5P was identified as the core molecule regulating migration and actin rearrangements in response to FGF1. Again, this work sheds some light on a localized regulation of PtdIns5P synthesis, possibly at the endosomal level (PIKfyve and MTMR3 being localized in this compartment) to regulate oriented migration 34, but again the effector(s) of the lipid remains to be identified. One interpretation is that PtdIns5P has the property of coordinating membrane and cytoskeleton dynamics (Fig. 4). These findings will undoubtedly stimulate future work to elucidate the molecular circuitry linking PtdIns5P to actin dynamics, vesicular trafficking, and cell migration in response to changes in the microenvironment.
PtdIns5P in the nucleus: Production and roles
A number of studies have described an independent phosphoinositide metabolism in the nucleus of various mammalian cells. Historically, the first PtdIns5P interacting protein discovered was the chromatin regulator ING2 14. It was therefore important to analyze the production and roles of PtdIns5P in the nucleus, even if its amounts are relatively low in this compartment. Figure 5 summarizes our current knowledge about this lipid in the nucleus.
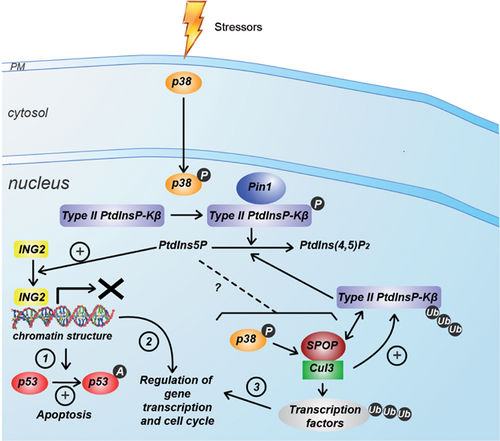
Type II PtdInsP-kinase β and p38-MAPK regulate the nuclear PtdIns5P pool
The metabolic routes involved in the production of nuclear PtdIns5P are still unknown but it has been clearly demonstrated that type II PtdInsP-kinases have a major role in the removal of this nuclear pool. These enzymes display different locations within the cell: type II PtdInsP-kinases α are cytosolic, while β is present both in the cytoplasm and nucleus, and γ in endomembrane compartments 3, 88. Evidence suggests that type II PtdInsP-kinase β is essential for modulating nuclear levels of PtdIns5P 3, 88. It was demonstrated that upon UV-irradiation, oxidative stress or etoposide treatment, p38-MAPK (Mitogen Activated Protein Kinase) becomes activated and phosphorylates type II PtdInsP-kinase β on Ser326, leading to inhibition of the enzymatic activity and nuclear accumulation of PtdIns5P in various cell types, such as MEL, HEK293, and HT1080 11. Because of the possibility of dimerization of type II PtdInsP-kinase α and β, it was postulated that the β isoform could act as a carrier to locate the more active α isoform into the nucleus upon stress. Although this possibility cannot be completely ruled out, only type II PtdInsP-kinase β was found to be inhibited by p38-MAPK, therefore suggesting that it is the activity of the β isoform itself that plays a role in the nucleus 89.
Given that PtdIns(4,5)P2 is the most abundant phosphoinositide in the nucleus, a potential interplay between the inhibition of type II PtdInsP-kinases and the activation of type I and II PtdIns(4,5)P2 4-phosphatases could take place to generate nuclear PtdIns5P 57. Even though nuclear PtdInsP2 phosphatase activity modification was not detected upon stress 11, the potential involvement of phosphatases in nuclear PtdIns5P production still remains to be thoroughly investigated.
While p38-MAPK/type II PtdInsP-kinase β are clearly implicated in the increase in nuclear PtdIns5P in MEL cells challenged by UV-irradiation 11, the increase in nuclear PtdIns5P elicited by H2O2 in U2OS cells seems to be largely independent of p38-MAPK 4. This suggests that ligand- or stress-dependent increase in PtdIns5P operate in different cell types. The relationship between the p38-MAPK pathway and PtdIns5P is more complicated than it appears at first glance. Recently, a new regulator of type II PtdInsP-kinases has been discovered: Pin1 is a proline isomerase that recognizes proteins phosphorylated on serine and/or threonine and regulates the localization and conformation (and thereby, in some cases, the enzymatic activity) of its targets. An elegant study has shown that Pin1 interacts with type II PtdInsP-kinases when phosphorylated by p38-MAPK upon oxidative stress, resulting in a decrease in kinase activity and a subsequent increase in nuclear PtdIns5P 90, 91. This process is important, because it induces expression of genes involved in increased viability of cells in response to oxidative stress. It suggests that nuclear PtdIns5P is a redox-dependent messenger regulating the sensitivity of cells to ROS in order to determine cell fate in response to oxidative stress – a process that might be of importance in inflammatory diseases and cancer 91. However, inhibition of type II PtdInsP-kinases only accounts for part of the PtdIns5P increase detected upon oxidative stress: unknown pathway(s) finely tune the levels of this lipid. It is also noteworthy that oxidative stress leads to PtdIns5P-dependent activation of Akt, which is only partly regulated by type II PtdInsP-kinases. Production of PtdIns5P and subsequent Akt activation could occur in the cytosol, and it is claimed that PtdIns5P and Akt could translocate together or separately into the nucleus 4. However, this is a far-fetched assumption, since no PtdIns5P transporter has been described.
Nuclear PtdIns5P regulates chromatin remodeling and gene expression
As previously mentioned, the chromatin regulator ING2 acts as a nuclear PtdIns5P receptor through its PHD domain 14. Stress-induced elevation of nuclear PtdIns5P causes translocation of ING2 to the chromatin-enriched matrix, where it triggers p-53 acetylation and apoptosis. A recent study 92, further highlights the outcome of the PtdIns5P/ING2 association by demonstrating that it regulates the repression of a subset of genes involved in DNA damage response. Fluctuations in nuclear and cytosolic PtdIns5P levels suggest that PtdIns5P could be produced at any of those locations, depending on the cell type and stress applied.
Interestingly, regulation of gene expression can occur through other PtdIns5P-binders. The PHD domain of the p62 subunit of the TFIIH transcription factor has been shown to have some specificity towards PtdIns5P, even though the effect of this association is unknown 93. In Arabidopsis, the trithorax-like factor ATX1 is a histone methyltransferase containing a PHD domain that interacts with PtdIns5P 94. Dehydration stress leads to an increase in PtdIns5P triggered by the plant homolog of myotubularin, AtMTM1. In this model, elevation of PtdIns5P in the cytosol sequesters ATX1 away from its nuclear targets, resulting in down-regulation of ATX1-dependent genes in response to dehydration 95. Again, it is possible that both cytosolic and nuclear signaling modules cooperate to achieve the adequate response to the initial insult. Another type II PtdInsP-kinase β-dependent response involving PtdIns5P production has been unraveled by the study of the type II PtdInsP-kinase β knockout mice. These animals display insulin-hypersensitivity. This phenotype could arise not only from the PtdIns5P-mediated activation of the Akt pathway – which is important for insulin-stimulated glucose uptake – but also from an alteration of insulin-dependent gene response according to reference 70.
Very recently, it has been shown that PtdIns5P is increased upon viral infection and induces type 1 Interferon production (regulator of the innate immune response) by binding to the transcription factor IRF3 (Interferon regulatory factor 3) and its upstream kinase TBK1 (TANK-binding kinase 1), thereby promoting TBK1-mediated IRF3 phosphorylation and activation 33. This work demonstrates that PtdIns5P-related signaling pathways are not only taken advantage of by bacterial pathogens, but that they are also involved in response to viral infections. This observation strengthens the notion that PtdIns5P is a regulator of major cellular processes.
Nuclear PtdIns5P and the Cul3-SPOP ubiquitin ligase
Another mechanism by which PtdIns5P can modulate nuclear functions is related to the regulation of ubiquitination of nuclear proteins. PtdIns5P elevation appears to stimulate the Cul3-SPOP ubiquitin ligase complex in a p38-MAPK-dependent way. Interestingly, type II PtdInsP-kinase β associates with SPOP in nuclear speckles, and can be ubiquitinated by Cul3, resulting in a down-regulation of the pathway that converts PtdIns5P into PtdIns(4,5)P2. In this model, p38-MAPK acts as a “producer” of PtdIns5P through inhibition of PtdInsP-kinase β. In a second step, PtdIns5P can activate p38-MAPK, leading to activation of the Cul3-SPOP ubiquitin ligase, and thereby reinforcing its own production. This novel pathway might be of importance in pathology, because one of the targets of Cul3-SPOP is the PDX-1 transcription factor, which plays key roles in pancreatic development and especially β-cells differentiation. These observations again suggest a link between PtdIns5P elevation and insulin response/diabetis 89, 96. Given our knowledge of the principal mechanism whereby ubiquitination regulates protein stability, location or activity, it would now be interesting to evaluate the extent to which PtdIns5P also targets cytosolic ubiquitination complexes.
Conclusions
Somewhat more slowly than the other phosphatidylinositol monophosphates (PtdIns4P and PtdIns3P), PtdIns5P is entering the gamut of lipid regulators involved in the control of cellular functions. Although the studies reported so far are relatively sparse and incomplete, two main functions of this lipid are emerging: one function is related to the pool of PtdIns5P in the nucleus, which can act as a regulator of chromatin organization via interaction with proteins such as ING2 to modulate gene expression in response to stress and DNA damage. Several gaps remain in our understanding of the origin of PtdIns5P in the nucleus: does it result from in situ production or transport? What is its physico-chemical presentation (membrane- or protein-associated)? What are its threshold levels and the molecular circuitry linking this lipid to the regulation of gene expression? Nonetheless, this research area has strong relevance for a variety of pathological processes including cancer.
The second function of PtdIns5P is related to its location in membranes facing the cytosol including plasma membrane, endosomal membranes and Golgi. The metabolism of PtdIns5P in these membranes appears to involve PIKfyve, myotubularins and type II PtdInsP-kinases. Furthermore, it is exploited by several pathogens such as bacteria and viruses. This lipid can modulate signaling mechanisms, including Akt activation, by altering membrane trafficking of active receptors. A current hypothesis is that PtdIns5P acts as a coordinator of membrane and cytoskeleton dynamics, but the characterization of its effectors remains elusive. A combination of genetic, biochemical imaging, and biophysical techniques is necessary to elucidate the precise physiological functions of PtdIns5P and its effectors. What we need to do is to determine which phosphoinositides do what, when, and where within the cell. In order to overcome technological barriers and further advance our knowledge on the role of PtdIns5P, the following developments are needed: the generation of a panel of specific bioprobes for PtdIns5P; the use of super-resolution imaging systems; the development of methods to locally change PtdIns5P levels. Furthermore, we will need to use model membranes and biophysical approaches to reproduce and analyze lipid/protein interactions and their consequences in different cell compartments.
Acknowledgements
This work was supported by grants from Inserm, Fondation ARC pour la Recherche contre le Cancer, Association Française contre les Myopathies, Fondation pour la Recherche Médicale, Association Lefoulon-Delalande, ANR 2010 MIDI 00703, ANR 2013 PI5PACTION.