Aging genomes: A necessary evil in the logic of life
Abstract
Genomes are inherently unstable because of the need for DNA sequence variation as a substrate for evolution through natural selection. However, most multicellular organisms have postmitotic tissues, with limited opportunity for selective removal of cells harboring persistent damage and deleterious mutations, which can therefore contribute to functional decline, disease, and death. Key in this process is the role of genome maintenance, the network of protein products that repair DNA damage and signal DNA damage response pathways. Genome maintenance is beneficial early in life by swiftly eliminating DNA damage or damaged cells, facilitating rapid cell proliferation. However, at later ages accumulation of unrepaired damage and mutations, as well as ongoing cell depletion, promotes cancer, atrophy, and other deleterious effects associated with aging. As such, genome maintenance and its phenotypic sequelae provide yet another example of antagonistic pleiotropy in aging and longevity.
Abbreviations
-
- CNV
-
- copy number variation
-
- DSB
-
- double-strand break
-
- ICL
-
- inter-strand crosslink
-
- NHEJ
-
- non-homologous endjoining
-
- SNP
-
- single-nucleotide polymorphism
Introduction
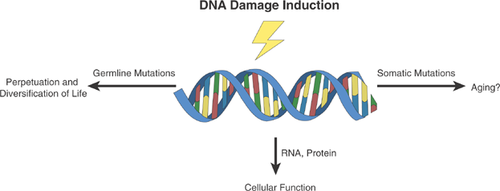
Structure and function of all organisms are ultimately determined by their genome, the complete set of species-specific hereditary information. For life to continue and survive the many unpredicted environmental fluctuations, genomes must have the capacity to change and reinvent themselves. Indeed, since the original emergence of life on earth 3–4 billion years ago, the fledgling genomes of the first replicators have now given rise through expansion and divergence to the current estimate of 30 million genomes, each representing a unique species. This is the product of genome instability, the tendency of genomes to undergo the changes termed mutations, i.e. addition, deletion, or substitution of bits of genetic code.
The ultimate driver of genome instability is DNA damage, physical alterations in nucleic acid structure, e.g. breaks, depurination, depyrimidination, crosslinks, and modified bases. DNA damage and its consequences are controlled by the complex machinery of genome maintenance, the network of protein products that repair damage in DNA, control cell division cycles, and signal programmed cell death and cellular senescence. The rudiments of genome maintenance can be traced back as far as the RNA-based ancestral cells 1.
The primary task of genome maintenance is twofold. First, it has to promote cell survival by quickly removing DNA damage, which is why genome maintenance was among the earliest selected genetic traits, possibly going back to the first replicators 2, 3. Second, through errors in its DNA transactions, genome maintenance provides the mutations necessary for evolution by natural selection. It is through mutations that Darwinian selection could lead to increasingly complex genomes and the adaptation of their hosts to the various challenges of a continuously changing environment.
With the emergence of multicellular organisms the role of genome maintenance became more complex. It now had to act not only to maintain the genome of the germline but also that of its somatic cells. While the germline genome needs to balance mutation frequency between genetic variation and genetic extinction, the somatic genome merely needs survival, at least until the reproductive period. Hence, one could argue that genome instability is a double edged sword. In the germ line it is needed to guarantee the perpetuation and diversification of life, while in the soma it could well be responsible for the end of life between germ lines (Fig. 1). Indeed, a relationship between longevity of the soma and the capacity of its genome maintenance systems to remove DNA damage has been proposed since the 1970s 4, 5, shortly after DNA repair had been discovered by Hanawalt 6, Setlow 7, and Howard-Flanders 8. By now it is well documented that defects in DNA repair increase cancer risk and can also lead to multiple early symptoms of aging other than cancer 9, 10. What we still do not know, however, is the role of DNA damage and mutations as causal factors in aging and how genome maintenance systems would encode longevity. Here, I will describe a conceptual framework as to how DNA damage could conceivably drive aging, at least in part, and how to test this.
DNA damage is abundant, but does it cause aging?
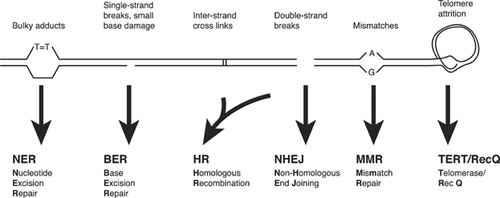
In contrast to RNA, which is reactive because of the extra hydroxyl group on its ribose sugar, DNA is a stable molecule that is unlikely to undergo spontaneous alterations. However, this situation is different under physiological conditions when ample hydrolysis, oxidation, alkylation, and many other spontaneous processes may lead to the introduction of a large variety of chemical lesions, varying from single- and double-strand breaks to depurination and depyrimidination, crosslinks, and base damage. Estimates vary, but it is possible that as many as tens of thousands of such lesions occur in each cell daily 11. In addition to spontaneous damage from endogenous sources, a fraction of that damage is caused by the environment, for example, pyrimidine dimers from sunlight, breaks and small base damage caused by ionizing radiation. Were it not for the many different, interconnected enzymatic systems to swiftly remove this damage and restore the original situation (Fig. 2 and 12), DNA could never be the repository of genetic information.
Spontaneous DNA damage in vivo is notoriously difficult to detect quantitatively, as illustrated by the many attempts to measure oxidative lesions. Direct evidence for the presence of oxidative DNA damage in cells in vivo has been obtained through the development of sensitive assays based on, for example, HPLC and gas chromatography. There are at least 20 known products of DNA oxidation, with 8-oxoguanine likely to be the most frequent one, comprising perhaps 5% of the total 13. While initially very high levels of 8-oxoguanine in animal tissues were reported (i.e. as many as three per 105 guanines), it is now clear that most of these lesions were artifacts of the experimental procedure to measure these lesions 14, 15. Increased precautions to prevent oxidation at various stages of sample preparation has now led to the assumption that the real level is likely to be in the order of several lesions per 107 guanines, which translates to less than a thousand per genome 16. For other oxidative lesions, such as thymine glycols, the levels are even lower 17. Indeed, even for apurinic/apyrimidinic (AP) sites, the most frequent spontaneous DNA lesion under physiological conditions, a steady state level of only about 23,000 per cell (corresponding to about four AP sites per 106 nucleotides) were found in human lymphoblastoid B cells 18.
Hence, while absolute figures of tens of thousands of lesions induced in cells each day suggest a very serious problem, it is difficult to see how steady state levels of a few lesions per 100,000 undamaged bases can contribute to the adverse effects that develop during the course of the aging process. At such low frequencies, with lesions continuously removed through highly sophisticated DNA repair pathways and re-introduced, it would be unlikely for a lesion to reside in a gene or its regulatory regions long enough to have adverse effects. Indeed, while there are many reports on age-related increases of various forms of spontaneous DNA damage in animal cells and tissues, results are often conflicting 19, 20. It seems a reasonable conclusion that DNA damage per se, even in the large absolute numbers of lesions induced in cells each day, is unlikely to ever reach levels that could play a direct role in causing age-related functional impairment and disease.
However, not all lesions are the same and a case can be made for certain, highly toxic lesions that could play an important role in aging. The best examples are DNA double-strand breaks (DSBs) and inter-strand crosslinks (ICLs). Both lesions are thought to be present at very low numbers, with estimates of about 50 DSBs per cell per cell cycle 21 and, possibly as little as 10 ICLs 22. Because they involve both DNA strands, repair of these lesions is difficult. It requires complex mechanisms, such as homologous recombination or, alternatively, a process called non-homologous endjoining (NHEJ) (Fig. 2 and 12). Especially NHEJ is not error-free and can lead to small deletions and chromosomal translocations 23.
DSBs have been associated with aging in a number of ways 24. First, γH2AX foci, generally considered a marker for DSB repair, accumulate in tissues of aging mice 25, 26. Second, mice harboring genetic defects in DSB or ICL repair show multiple symptoms of aging very early in life 27, 28. Third, DSBs were found increased with age in human and mouse oocytes, which was associated with a decline in the expression of genes involved in DSB repair, i.e. Brca1, Rad51, and ATM. Importantly, women with heritable mutations in BRCA1 are not only at a much greater risk for breast cancer, they also produce fewer oocytes in response to ovarian stimulation; the same was observed for mice with heterozygous mutations in Brca1 29. Finally, a data mining approach suggested non-homologous end joining of DSBs as an especially important DNA repair pathway for aging 30.Taken together, these results strongly suggest a pro-aging role of DSBs and, possibly, related highly toxic lesions, such as ICLs.
How can such low amounts of DNA damage drive aging? Somewhat surprisingly, the main culprit may be genome maintenance itself.
The Janus face of genome maintenance
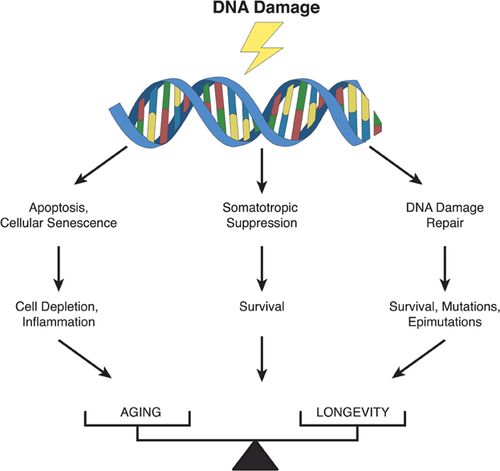
Figure 3 schematically depicts a complete picture of genome maintenance in a multicellular organism, which can roughly be divided between DNA repair per se and the cellular responses to DNA damage. Genome maintenance has been studied mainly in cultured cells and it remains poorly understood how DNA damage responses function in vivo and maintain tissue functionality. What we do know is that defects in genome maintenance pathways cause complex disease phenotypes characterized by developmental failure, cancer susceptibility, and premature aging 31. Genome maintenance has since long been considered as a longevity assurance system and a possible determinant of species-specific life span 32. While the evidence for such a relationship remains inconclusive, there is indeed a trend towards higher DNA repair activities in cells from longer lived species. For example, cells from short-lived rodent species have a much reduced capacity to remove UV-induced pyrimidine dimers from their DNA as compared to human cells 5, 33. Similarly, the activity of poly(ADP-ribose) polymerase 1 (PARP-1), involved in base excision repair and possibly other repair pathways, in white blood cells of different mammalian species correlates with maximum life span 34. Moreover, the levels of DNA-dependent protein kinase, which plays a key role in the repair of DNA double-strand breaks, correlate well with species-specific life span 35.
Other evidence suggesting that longer-lived species do have superior genome maintenance systems compared with shorter-lived species is the more rapid rate of DNA sequence evolution in short-lived rodents compared with the corresponding evolution rate in longer-lived primates; conceivably, this difference could be due to superior genome maintenance in the longer-lived species 36. Finally, a mouse cell in culture has a much higher probability of undergoing karyotypic changes and neoplastic transformation than does a human cell 37, 38. This may be related to a difference in telomere biology, a critical component of genome maintenance. Mouse cells have very long telomeres, and in contrast to the situation in human cells, telomerase activity of mouse cells in culture is not repressed. Thus, tumor cells, which require telomerase activity, can more easily progress in mice than in humans. In humans, the absence of telomerase activity in most somatic cells leads to telomere shortening, which signals cellular senescence, a major anticancer mechanism 39.
There is some evidence that activities of genome maintenance systems decline somewhat with age 40-42, but perhaps more importantly there is evidence that DNA repair is more active in stem cells and during early development than in normal, somatic cells in adult organisms. For example, there is evidence that mice stem cells have a significantly lower frequency of spontaneous mutations than normal somatic cells, most notably reduced loss of heterozygosity events, a result of mitotic recombination 43. Indeed, embryonic stem cells appear to be superior in homologous recombination repair 44, possibly because homologous recombination repair, which is relatively error-free, is the preferential pathway for the repair of DSBs during early development 45. On the other hand, in Drosophila male germ cells the utilization of homologous recombination was reported to increase with age with NHEJ less utilized 46. It was speculated that the error prone, faster NHEJ pathways would be preferred in young individuals requiring rapid growth, with the more error-free, slower HR pathway favored at later ages, when growth is no longer an issue. Whichever of these possibilities is correct, and this may depend on the species or environmental conditions, alterations in the utilization, and/or activities of DNA repair pathways during development and aging are likely to take place. However, a decline in genome maintenance activities is not required to still see adverse effects of DNA damage.
When facing a burst of DNA damage or higher than normal levels of highly toxic DNA lesions, such as DSBs or ICLs, the cell may respond in a way that will maintain somatic genome integrity, but at the cost of cell depletion late in life. The best known of such responses to DNA damage are apoptosis (programmed cell death) and cellular senescence (irreversible cell cycle arrest). Apoptosis is essential for the generation of multicellular tissues during embryonic development as well as the maintenance of cellular homeostasis 47. Increased apoptosis can contribute to disease, including neurodegenerative disease and atherosclerosis. However, apoptosis also serves as a defense system against cancer by eliminating cells at risk for neoplastic transformation due to excessive DNA damage. While initially beneficial, over the long-term apoptosis can result in tissue atrophy, a well-documented aging phenotype.
Like apoptosis, cellular senescence is antagonistically pleiotropic, since it suppresses cancer at early age, but at the cost of promoting aging at later ages, for example, by exhausting progenitor or stem cell reservoirs 48, 49. Like apoptosis, senescence can act as a DNA damage response and is induced in cultured cells by various mutagenic agents, most notably agents that cause genome rearrangement mutations, such as ionizing radiation or hydrogen peroxide. Interestingly, in human diploid fibroblasts, senescent cells were found to secrete inflammatory cytokines 50, which would add inflammation, a major age-related health problem, to the late-life adverse effects of genome maintenance.
Not all responses to DNA damage are associated with adverse effects. For example, a recently discovered component of the DNA damage response is the downregulation of the insulin/insulin-like growth factor-1 signaling (IIS) pathway. Mice deficient for certain DNA repair pathways display a host of premature aging symptoms, live shorter and show dampened IIS 51, 52. Interestingly, dampened IIS signaling due to single-gene mutations in components of the IIS nutrient-sensing network have been identified that extend normal life span in multiple species, possible by metabolic reprogramming to reduce damage and/or upregulate cellular defense 53. Similarly reduced IIS signaling in long- and short-lived mutant animals seems paradoxical, as they represent both extremes of aging. It has been suggested that reduced IIS signaling in this context is a survival response, which if constitutionally present would increase normal life span also 9, 10. Such metabolic reprogramming would be the opposite of what is often seen in cancer, i.e. the upregulation of IIS and TOR signaling to promote growth 54.
Errors in genome maintenance are inevitable and irreversible
While extraordinarily efficient in resisting the avalanche of chemical changes threatening the genome's integrity, genome maintenance is not perfect. Errors during DNA damage processing, in both dividing and non-dividing cells 55, can lead to mutations. While the frequency of such mutations may be remarkably low as compared to the large numbers of lesions induced in our genome every day, they are irreversible and, therefore, could conceivably accumulate to a level where they exert adverse effects. (The same is true for epimutations, e.g. random alterations in DNA methylation or histone modification patterns 56.) Mutation accumulation in the genome of somatic cells should not be confused with mutation accumulation in the germline. Mutations in the germline are heritable and can become subject to natural selection. Indeed, building on previous work by Fisher 57, Haldane 58, and others 59, Medawar 60 was the first to explicitly consider aging as a result of the late effects of deleterious alleles. There is no selection against the germline mutations that lead to such deleterious alleles as long as their effects are restricted to late stages of life and do not affect early survival or reproductive success. Reduced selection efficacy post-reproductively also explains why cellular defense systems in the soma, such as genome maintenance, are imperfect. There would be no selective advantage of suppressing somatic mutagenesis for much longer than the age of first reproduction.
DNA mutations accumulating in the somatic cells have been hypothesized as a major cause of aging at least since the 1950s by Failla 61 and Szilard 62. Because somatic mutations are usually present at low frequencies, spread throughout the genome, their quantification, and characterization is difficult. When the mutation is known it is possible to directly confirm its presence, even at low abundance, for example, using real-time PCR. In such cases it is often possible to detect one mutant template in the presence of 1,000 wild type templates 63. But such methods are of little use when the mutations are unknown as is the case with possible age-accumulated mutations in different organs and tissues. Indeed, methods that can detect unknown mutations in genomic DNA isolated from a tissue or pool of cells can only identify those that occur at a very high frequency at a particular site. An exception is molecular cytogenetics, which is able to identify very large genomic changes (typically no smaller than 1–5 million basepairs) at the single cell level. Studies using this technology did reveal an age-related increase in chromosomal aberrations in lymphocytes, both in mice 64, and in humans 65. More recently, it has become clear that chromosomal mutations, such as aneuploidy, can affect as many as 1–2% of all cells in a tissue for each chromosome, even in a mostly postmitotic tissue such as the brain 66, 67.
Experimental access to low-abundant mutations can be obtained using reporter systems. For example, using the classic hypoxanthine phosphoribosyltransferase (HPRT) gene as an endogenous reporter it has been shown that mutations accumulate with age in blood lymphocytes of both humans and mice 68, 69. However, this type of assay only allows analyzing cells that are mitotically active and can be cloned. To get access to all organs and tissues of an aging organism, we developed transgenic mice harboring the bacterial lacZ reporter gene that can be excised from chromosomal DNA and subsequently screened for mutations in a bacterial host 70, 71. These transgenic reporter assays revealed an age-dependent accumulation of somatic mutation loads in a variety of organs and tissues 72-74. Both the rate of this increase and the mutation spectra differed greatly from organ to organ; especially the large fraction of genome rearrangements vis-à-vis point mutations is noteworthy because of the potentially large effects of the former, even at very low frequencies 75.
More recently, a lacZ-reporter model, similar to the mouse model, was constructed in Drosophila melanogaster 76. Also in flies, significantly higher somatic mutation loads were observed in the old as compared to the young animals. Interestingly, the spontaneous mutation frequency in somatic tissues from flies, on a per locus basis, is about three times higher than in the mouse, with the far majority of the mutations being genome rearrangements 77. Flies may be able to sustain a much higher level of somatic mutations due to the structure of their genome, which is smaller and much more compact than that of mammals, with fewer long-distance regulatory interactions between genes 78.
Most reporter systems indicate a somatic mutation frequency that varies from about 1 × 10−5 to 1 × 10−4 in tissues, such as liver or spleen, on a per locus basis. For the lacZ reporter gene of about 3 kb this would correspond to about 20–200 mutations per diploid cell. With the emergence of next-generation sequencing it has now become feasible to compare different generations of animal species, including humans, and directly determine germ line (rather than somatic) mutation rates. In a recent study, in which the genome-wide loads of de novo mutations were determined in 78 Icelandic parent-offspring trios by whole genome sequencing, an average number of about 60 de novo mutations were found per trio, with the number of paternal mutations substantially higher than the mean number of maternal mutations and increasing with the father's age 79. This corresponds to a germline mutation rate of 1.2 × 10−8 per nucleotide per generation. The Icelandic study was limited to single nucleotide mutations and did not include other types of mutations, such as structural variation. For example, copy number variation (CNV) has a germ line mutation rate in the human genome of up to 1 × 10−4 mutations per generation, with the frequency of very large (>100 kb) CNVs as high as 1.2 × 10−2 per generation 80, 81. In the mouse, this situation proved very similar, with the rate of CNVs of approximately 4 kb to 4 Mb found to vary from 3.6 × 10−3 to 1.1 × 10−2 per generation 82. Of note, the rates of change varied about four orders of magnitude across different loci, with many CNVs arising multiple times within distinct lineages.
It is difficult to extrapolate these germline mutation rates to somatic cells because there is evidence that the germline is better protected from DNA damage than the soma. Indeed, base excision repair rates of various lesions in human or rat male germ cells were found to be markedly higher compared to blood lymphocytes or liver hepatocytes 83. This is consistent with the low mutation frequencies observed in male germ cells using the aforementioned mutational reporter genes 84.
Similar assay systems as used for detecting germ line CNVs were also used for analyzing blood and tissue DNA from individuals of different ages for somatic mutations. As mentioned, in such direct assays only mutations affecting a substantial fraction of cells, due to expansion of a single, de novo mutation, can be detected. Nevertheless, in this way age-related accumulation of copy number variants could indeed be detected 85-88. Of course, this merely reflects the tip of the iceberg, with the true number of low-abundant somatic variants arising during aging far higher.
Similar to germ line mutation rates also somatic mutation rates were found to differ among different types of genomic sequence. Indeed, in regions containing repeat elements, such as mini- and micro-satellites, retrotransposons, and telomeres, spontaneous mutations can occur at a much higher rate than in single-copy regions. Mutations at microsatellite loci have been found to occur at rates as high as 1 × 10−2 per locus in human blood and increase with age 89. Retrotransposition has been observed in rodent brain in vivo 90, and deep sequencing has revealed insertions of retrotransposons in Drosophila and human brain 91, 92. Telomeres, regions of repetitive DNA protecting chromosome ends from deterioration, significantly shorten with age in mammalian cells and tissues 93, in part due to the end replication problem 94.
Finally, with the emergence of next-generation sequencing it has become possible to sequence whole exomes or even whole genomes from human cancers. Interestingly, the number of somatic mutations in tumors appeared to be significantly higher when the tumor was derived from an old patient as compared to a young one 95, 96. Indeed, mathematical modeling strongly suggests that half or more of somatic mutations in tumors arise before initiation of the tumor, i.e. during development and aging 97.
Hence, it seems safe to conclude that various types of DNA mutations, from aneuploidy and other gross chromosomal changes to copy number variation, small deletions, and basepair substitutions, inevitably and irreversibly, accumulate with age. What we do not know is the landscape of somatic mutations in an aged tissue and if this could explain, at least in part, age-related functional decline and disease.
Towards a comprehensive map of age-related somatic variants
From the above we may conclude that the average number of cumulative mutations in tissues of an aged organism is surprisingly high 98. In contrast to DNA damage, which is continuously induced and rapidly repaired, DNA mutations are irreversible. The question is if somatic mutation frequency and spectrum at old age are severe enough to cause aging phenotypes other than cancer. Cancer arises from single cells as a consequence of repeated cycles of mutagenesis and selection for growth advantage, tissue invasion, apoptosis suppression, and many other attributes that promote tumor progression 99. Loss of proliferative homeostasis, giving rise to hyperplasias, benign neoplasms, and malignant neoplasms, is a major component of the aging phenotype and likely to be causally related to age-related mutation accumulation in cells from self-renewing tissues 100. By contrast, a causal role of mutations in tissue functional decline would rely on a cumulated mutation load in many or most cells of an organ or tissue that is high enough to have adverse effects without selection. How could random mutations affect function when the chance of them ever hitting the protein-coding region of a gene is small?
The specialized functions carried out by differentiated cells are not based on single genes, but involve extensive networks of genes and various non-coding, regulatory sequences 101, 102. There are many ways of disrupting such networks and any mutation hitting a sequence that is part of the network can affect its function. Moreover, gene functional networks are connected to many others and mutations affecting a network can have ripple effects, for example, by deregulating epigenetic processes 103, 104. Hence, with such extensive genomic targets it is conceivable that an age-related, accumulation of somatic mutations, even when random, can adversely affect the specific functions carried out by the different cell types in an organism, producing phenotypic effects that are consistent from individual to individual, across generations and even across multiple species. The question is if enough cells in a tissue are affected to a significant extent to cause functional decline. This depends on somatic mutation frequency and spectrum at old age.
In principle, somatic mutations could be analyzed by whole genome sequencing. Indeed, whole genome sequencing has now provided us with entire, decoded genomes of thousands of individuals 105. Somatic mutations, even when occurring at very low frequencies, should be detectable by sequencing thousands or tens of thousands of times across the genome and call all possible variants of the consensus sequence. However, in practice this would be very inefficient and expensive, and also essentially constrained by the high rate of sequencing errors, which can amount to close to 1% 106, 107. Indeed, somatic mutations would affect only one or very few of the sequencing reads obtained from a particular locus (Fig. 4). Note that this is different from polymorphic differences among individuals, which would affect about half of all reads for that locus (a heterozygous single-nucleotide polymorphism or SNP; Fig. 4). When sequencing bulk DNA from the entire cell population or tissue, genuine somatic mutations cannot be distinguished from sequencing errors, which are scattered through the reads (Fig. 4). To some extent it is possible to correct for sequencing errors, for example, by independently sequencing each of the two strands of a DNA duplex; true mutations are found at the same site in both strands, while sequencing errors typically affect only one strand 108. However, truly deep sequencing to detect all possible mutations across the genome remains unfeasible. One possible solution for this problem is to sequence single cells after whole genome amplification 109. After amplification, mutations unique for each cell will now occur in half of all reads for a particular locus. Only half of all reads, because a true mutation will affect one allele only, since it is highly unlikely that the other allele will also be hit at that same site (Fig. 4). The feasibility of this approach was demonstrated by the detection of a significant elevation in mutation frequency in cells treated with a powerful mutagen 109.
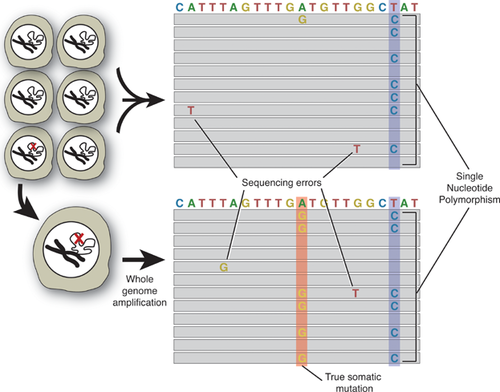
Single-cell sequencing would allow us to resolve the mosaic landscape of the somatic genome as this evolves during development and the aging process. It is now generally accepted that postzygotic mutations can cause functional decline and disease 98, 110-112. Genome instability over time essentially turns an aging tissue into a mosaic of cells with different genotypes. Somatic mosaicism for mutant disease genes is obvious in disorders where the manifestations are readily seen, e.g. the skin in type I neurofibromatosis 113. De novo mutations have also been found as a frequent cause of sporadic autism 114 and schizophrenia 115 as revealed by genomic sequencing. Evidence for widespread somatic copy number mosaicism was obtained for human skin 116. Single-cell sequencing would directly resolve intra-tissue heterogeneity, identifying unique and recurrent mutations in different cells and cell types. Most importantly, single-cell genomics would identify complete sets of mutations for a representative number of cells in different organs and tissues. This would allow, for the first time, accurate predictions of the functional impact of random mutations in aging tissues and organs.
Conclusions and prospects
Since its emergence in the first replicators, genome maintenance has been acting as a two-edged sword, promoting survival of some at the cost of genetic extinction of many. In multicellular organisms early benefits in terms of high rates of cell growth and cancer suppression are at the cost of depletion of functional cells, accumulating senescent cells, and increased somatic mutations, later in life. In this sense, genome maintenance is a typical example of antagonistic pleiotropy. Late in life, when the forces of natural selection have become weak, the postponed adverse effects of genome maintenance become progressively manifest. It is likely that genetic loci controlling these processes also control life span by balancing beneficial and detrimental effects as a function of age 98.
This antagonistic pleiotropic model of genome maintenance as a determinant of aging is visualized in Fig. 5. Among areas depleted from cells, with increased numbers of senescent cells and neoplastic or hyperplastic lesions, accumulated mutations adversely affect functional genetic networks by inactivating protein-coding and/or gene regulatory sequences 98. Hence, this would convert the random process of mutation accumulation into systematic effects. Of note, essentially the same is true for random alterations in the epigenome, the set of modifications to DNA and proteins that determine gene activity. In contrast to DNA sequence changes, systematic changes in the epigenome occur frequently, for example, during development, but also as part of normal processes in adult life and during aging 56. These controlled processes should be distinguished from epimutations, i.e. irreversible errors in the restoration of DNA methylation and/or histone modification patterns after replication of repair. Like DNA sequence changes, epimutations are irreversible and accumulate with age.
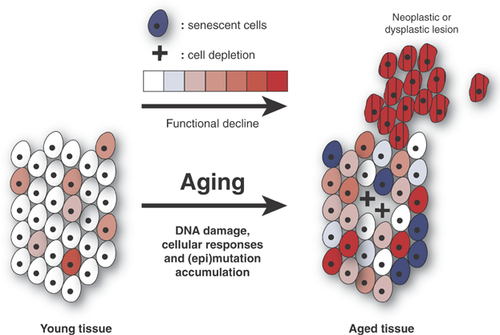
Using single-cell approaches, in combination with deep sequencing, it seems likely that the comprehensive landscape of age-related, somatic mutagenesis will be resolved soon enough. The major challenge that remains is to develop interventions that promote healthy aging by re-balancing genome maintenance towards attenuating, arresting or reversing its late-life, adverse effects while maintaining or enhancing its beneficial role throughout life. A strategy to achieve this requires at least three components. First, we need to develop approaches to stimulate cell and tissue regeneration to make up for depleted stem cell reservoirs. Recent spectacular progress in embryonic and induced pluripotent stem cell technology provides at least a scaffold for beginning to do that 117. Second, it will be necessary to eliminate accumulated senescent and neoplastically transformed cells from aged tissues. Also here recent progress has been made. In one approach, senescent cells were eliminated from mouse tissues based on expression of p16 as a marker 118. Removal of neoplastic cells depends on progress in cancer therapy. Recent developments in targeted therapy and immunotherapy offer some hope that such a feat can in fact be accomplished over the next decades 119, 120. Finally, we will need to manipulate genome maintenance systems to progressively increase error-free repair, thereby delaying or preventing mutations from accumulating. This last component is the most difficult to achieve because, first, we do not as yet know enough about the mechanisms that determine the error level that is associated with various DNA repair pathways; and, second, this will require the extensive utilization of extraordinarily specific genome engineering tools. To some extent these are now available in the form of Talen and Crispr 121. Hence, it seems reasonable to anticipate that over the next decade novel interventions will be developed that will allow us to diminish age-related genotoxic stress and optimize genome maintenance for functioning at old age.