Multi-Omics Insights Into the Role of Fructooligosaccharides Supplementation in Alleviating Salpingitis in Laying Hens
Funding: This study was supported by the Research Fund for National Non-profit Institutions (Grant ZX2517), and the China Agriculture Research System of MOF and MARA (CARS-40-S20).
ABSTRACT
Salpingitis is a highly prevalent disease that reduces production performance and egg quality in laying hens, severely impeding the sustainable development of the egg-laying industry. Fructooligosaccharides (FOS) play a significant role in regulating gut health and immune function. However, the mechanisms by which FOS alleviates salpingitis remain unclear. This study aimed to elucidate how FOS mitigates salpingitis using multi-omics approaches. A total of 270 34-week-old Hy-Line Brown laying hens were randomly assigned to three groups: a control group with a basal diet (CN), a lipopolysaccharide (LPS)-challenged group on a basal diet (CN_LPS), and an FOS-supplemented group (1 g/kg diet) with LPS challenge (FOS_LPS). The results showed that the supplementation of FOS significantly ameliorated LPS-induced inflammation and atrophy in the magnum of hens (p < 0.05). The mRNA expression levels of TLR2, MYD88, NF-κB, and COX2 in the FOS_LPS group were significantly reduced in the magnum compared to the CN_LPS group (p < 0.05). In contrast, the expression of ABCA9, BIRC5, and MYRF genes was significantly higher in the FOS_LPS group than in the CN_LPS group. Compared to the CN_LPS group, the FOS_LPS group exhibited a reduction in the abundance of Rikenellaceae_RC9_gut_group and Alistipes, whereas the abundances of Lactobacillus, Ruminococcus_torques_group, and Phascolarctobacterium were increased in cecal chyme. In addition, the FOS_LPS group exhibited elevated relative concentrations of S-lactoylglutathione and thymol sulfate in plasma as compared to the CN_LPS group. Collectively, FOS mitigated LPS-induced salpingitis by modulating key inflammatory pathways, restoring gut microbiota (e.g., increased Lactobacillus, decreased Rikenellaceae), and enhancing metabolic homeostasis.
1 Introduction
The poultry industry has effectively provided an affordable, high-quality source of protein to meet the growing global demand for animal-derived food [1, 2]. Eggs are widely recognized as a highly nutritious source of protein for human consumption [3]. In 2021, China accounted for 34.4% of global egg production, underscoring its substantial role in meeting worldwide demand [4]. Concurrently, there has been an increasing concern over the quality of eggs [5]. Egg formation occurs as the yolk traverses through the oviduct, and the health of the oviduct directly influences egg quality [6].
Salpingitis, a prevalent disease in large-scale laying hen operations, primarily results from bacterial infection and stress [7]. The main symptoms of salpingitis in laying hens include reduced egg production, shortened or absent peak laying periods, and poor eggshell quality, as well as thin, soft, and sandy-shelled eggs [8]. The frequent occurrence of salpingitis leads to declines in both egg production and quality, causing substantial economic losses to the poultry industry [8]. Existing research studies suggest that the decline in albumen quality is intricately linked to dysfunctional oviduct activity. This is primarily evidenced by the downregulation of critical genes responsible for protein synthesis and secretion, alongside structural alterations such as the degradation of β-ovomucin subunits and the cleavage of O-glycosidic bonds [9, 10]. Current treatment and prevention of salpingitis primarily rely on antibiotics, which pose a risk of drug residues in animal products and may threaten human health. Therefore, nutritional strategies for the prevention of salpingitis have become a critical focus in poultry management.
Fructooligosaccharides (FOS), a class of indigestible carbohydrate with well-established prebiotic properties, are recognized as one of the top three prebiotics worldwide [11]. Multiple studies have demonstrated that dietary supplementation with FOS can significantly improve performance and egg quality of laying hens [12], enhance intestinal morphology of broilers [13], and modulate gut microbial composition in mice [14]. Obianwuna et al. [15] reported that FOS supplementation improved laying performance, egg quality (as measured by Haugh unit and albumen height), amino acid digestibility, immune response, and antioxidant capacity in laying hens. Additionally, Andrade et al. [16] showed that FOS alleviated mucositis by modulating the gut microbiome. Similarly, Mohammed et al. [17] found that FOS reduced placental inflammation in hamsters by suppressing neutrophil infiltration and the expression of pro-inflammatory cytokines. However, whether FOS can alleviate salpingitis in laying hens remains unclear.
Lipopolysaccharide (LPS), a major bioactive component of the outer membrane of Gram-negative bacterial, is widely used as a primary modeling agent to induce acute inflammatory responses and muscle tissue damage in experimental studies [18]. In recent years, advances in high-throughput technologies have facilitated a deeper understanding of molecular mechanisms and disease pathogenesis [19]. Consequently, the use of integrated multi-omics approaches has grown in popularity, as these methods reduce experimental and biological biases [19]. For instance, Xu et al. [20] demonstrated the key role of the gut microbiota-ovary axis in regulating follicular development in Meishan and Landrace × Yorkshire sows using a multi-omics analysis. Therefore, we hypothesized that FOS modulates the cecal microbiota, alters plasma metabolite profiles, and subsequently regulates the expression of inflammation-related genes in the oviduct, thereby preventing salpingitis. The objective of this study was to investigate the protective mechanisms of FOS in LPS-induced salpingitis in laying hens through an integrated analysis of the oviduct transcriptome, plasma metabolome, and cecal microbiota.
2 Materials and Methods
2.1 Experimental Materials
FOS (347 Da, DP: 2–4), primarily composed of 1-Kestose, Nystose, and 1F-Fructofuranosylnystose with a combined content of 95%, was procured from Baolingbao Biology Co. Ltd. (Shandong, China) [21]. The Lipopolysaccharide (LPS, Cat# L4005-500MG) derived from E. coli O55:B5 was pruchased from Sigma Aldrich (Shanghai, China).
2.2 Experimental Design and Sample Collection
A total of 270 34-week-old Hy-Line Brown laying hens were randomly assigned to three groups: (1) control group fed a basal diet (CN), (2) LPS-challenged group on a basal diet (CN_LPS), and (3) FOS-supplemented group (1 g/kg diet) with LPS challenge (FOS_LPS). Each group comprised six replicates with 15 birds per replicate. After a 24-week feeding period, 18 laying hens per group were randomly selected, and salpingitis was induced by placing a 2 mg/kg body weight LPS capsule into the vaginal region. LPS dosage was adjusted per body weight and administered via a medical capsule. Hens were restrained with legs secured and body inverted to expose the cloaca for precise delivery. Twelve hours post LPS challenge, six laying hens per group were randomly selected for blood collection from the wing veins. Plasma was separated by centrifugation (4°C, 3000 rpm, 10 min) and stored at −20°C. Hens were then euthanized using carbon dioxide (CO2), and oviducts were immediately dissected. One tissue sample (1 cm2) was fixed in 10% neutral buffered formalin for histopathological analysis, whereas the other was immediately snap-frozen in liquid nitrogen and stored at −80°C for subsequent RNA sequencing. Fresh cecal chyme was collected for microbial composition analysis [22].
2.3 Diets and Management
Diet formulation followed the NRC [23] recommendations. The composition and nutrient concentrations of the basal diet were outlined in Table 1. The laying hens were housed in a 3-tiered cage system (3 hens per cage), maintained under 16 h light: 8 h dark cycles at 21°C–24°C, and vaccinated per standard protocol. All birds had ad libitum access to feed and water.
| Items | Content |
|---|---|
| Ingredients | |
| Corn | 61.00 |
| Soybean meal | 25.58 |
| Soybean oil | 1.80 |
| CaHPO4 | 1.90 |
| Limestone | 9.00 |
| NaCl | 0.15 |
| Choline chloride (50%) | 0.20 |
| Premixa | 0.22 |
| DL-Met | 0.10 |
| L-Lys·HCl | 0.05 |
| Total | 100.00 |
| Nutrient levelsb | |
| ME/(MJ/kg) | 11.41 |
| CP | 16.50 |
| Ca | 3.64 |
| TP | 0.63 |
| Lys | 0.81 |
| Met | 0.35 |
- a The premix provided the following per kg of diets: VA 9500 IU, VB2 6 mg, VB12 0.025 mg, VD3 62.5 μg, VE 30 IU, VK3 2.65 mg, biotin 0.0325 mg, folic acid 1.25 mg, D-pantothenic acid 12 mg, nicotinic acid 50 mg, Cu 8 mg, Fe 80 mg, Mn 100 mg, Zn 75 mg, I 0.35 mg, Se 0.15 mg.
- b The total CP values were measured in the mixed feed, with each value determined in triplicate. Other nutrient compositions are calculated.
2.4 Histological Assessment
Formalin-fixed magnum tissues were paraffin-embedded, sectioned (4 μm), and stained with hematoxylin and eosin (HE). Slides were examined under a BX43 microscope (Olympus, Tokyo, Japan) at a magnification of 100x magnification [24]. The degree of tissue damage was evaluated using histological scoring methods for glandular atrophy and inflammation. The scoring criteria used in this study were modified from the original publications by Francis-Malave et al. [24] and Liu et al. [25]. The scoring criteria for glandular atrophy were based on the percentage of the entire lamina propria affected by atrophy, with a score of 0 indicating no atrophy and scores of 1–4 representing less than 25%, 25%–50%, 50%–75%, and greater than 75% involvement, respectively. The pathological scoring criteria for interstitial inflammation were as follows: 0 = absence of inflammatory cell infiltration; 1 = mild, presence of a small number of lymphocytes infiltrating; 2 = moderate, capillary congestion, presence of a limited amount of lymphocyte infiltration, occasional inflammatory lesions in the local lamina propria; 3 = severe, capillary congestion, significant lymphocyte infiltration and a few inflammatory lesions. The histological scores were determined by summing the scores for glandular atrophy and inflammation.
2.5 Cytokine Analysis
The concentrations of cytokines IL-10 (interleukin-10, Cat#: MK5023A), IL-6 (interleukin-6, Cat#: MK2661A), and IFN-γ (Interferon-γ, Cat#: MK2660A) were quantified using chicken-specific ELISA kits (Jiangsu Sumeike Biological Technology Co., Ltd) in accordance with the manufacturer's protocols [26].
2.6 Real-Time PCR
RNA was extracted from the magnum of the oviduct and subsequently subjected to qPCR analysis. Total RNA was extracted from magnum tissue utilizing the RNA Easy Fast Tissue/Cell Kit DP451 (Tiangen Biotech Co. Ltd., Beijing, China). The RNA quality was evaluated by determining the OD260/OD280 and OD260/OD230 ratios, as described by Liu et al. (2023b). Subsequently, 1 μg of the extracted RNA was reverse-transcribed into complementary DNA (cDNA) using the PrimeScript RT Reagent Kit RR047A (TaKaRa, Dalian, China). Quantitative PCR (qPCR) analysis was then conducted employing the TB Green Premix Ex Taq Kit RR420A (TaKaRa, Dalian, China). A 25 μL reaction mixture was prepared containing 12.5 μL of TB Green Premix Ex Taq (Tli RNaseH Plus) (2×), 0.5 μL of PCR forward primer (10 μM), 0.5 μL of PCR reverse primer (10 μM), 2 μL of DNA template, and 9.5 μL of nuclease-free water. The following thermal cycling protocol was programmed on the real-time PCR instrument: initial denaturation at 95°C for 30 s; 40 cycles of denaturation at 95°C for 5 s, annealing at 60°C for 30 s; followed by a melt curve analysis (65°C–95°C with 0.5°C increments). At the conclusion of the qPCR reaction, a melting curve analysis was performed to verify the specificity of the amplified products. The 2−ΔΔCt method was utilized to determine the relative mRNA expression levels, with β-actin gene expression serving as the normalization control [17]. The primer sequences employed for real-time PCR can be found in Table S1.
2.7 Transcriptome Analysis of the Magnum of the Oviduct
Total RNA was isolated from the tissue utilizing TRIzol Reagent (Invitrogen) in accordance with the manufacturer's guidelines, and genomic DNA was eliminated using DNase I (TaKara). The quality of the RNA was evaluated with the 2100 Bioanalyzer (Agilent Technologies), and its concentration was determined using the ND-2000 spectrophotometer (NanoDrop Technologies). RNA purification, reverse transcription, library construction, and sequencing were executed at Shanghai Majorbio Bio-pharm Biotechnology Co. Ltd. (Shanghai, China), following the manufacturer's protocols (Illumina, San Diego, CA). The transcriptome library was constructed using the TruSeq RNA Sample Preparation Kit (Illumina, San Diego, CA) with 1 μg of total RNA. Post-quantification via TBS380, the paired-end RNA-seq library underwent sequencing on the Illumina NovaSeq 6000 platform, employing a 2 × 150 bp read length. The raw paired-end reads were trimmed and subjected to quality control using fastp with default settings. Subsequently, clean reads were aligned to the chicken genome (GRCG7B, https://www.ncbi.nlm.nih.gov/genome/111) in orientation mode using HISAT2. To identify DEGs (differentially expressed genes) between two different samples/groups, the expression level of each gene was calculated according to the transcripts per million reads (TPM) method. RSEM was used to quantify gene abundances. The identification of differentially expressed genes (DEGs) was performed using the DESeq2 software, with DEGs defined by the criteria of |log2FC| ≥ 1 and p value < 0.05, and visualized using a volcano plot in R language. Additionally, multiple groups were compared, and the Kruskal–Wallis H test method was employed to further screen for DEGs. After identifying DEGs, we utilized the KOBAS tool to perform enrichment analysis of KEGG Pathway (http://kobas.cbi.pku.edu.cn/home.do) [27]. Finally, the DEGs identified in this study were subjected to cluster analysis.
2.8 Cecal Microbiota
The microbial communities were analyzed using high-throughput amplicon sequencing of the 16S rRNA. A comprehensive description of this methodology is provided by Feng et al. [28]. Total DNA was extracted from cecal microbial communities utilizing the FastDNA Spin Kit for Soil (MP Biomedicals, CA, USA), following the manufacturer's protocol. The quality of the extracted DNA was evaluated via 1% agarose gel electrophoresis, and its concentration and purity were measured using a NanoDrop2000 spectrophotometer. The V3-V4 hypervariable regions of the bacterial 16S rRNA genes were amplified using universal primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). The PCR products were verified through 2% agarose gel electrophoresis and subsequently purified according to the instructions provided with the AxyPrep DNA Gel Extraction Kit. The purified amplicons were quantified using a Quantus Fluorometer. Library preparation was conducted using the NEXTFLEX Rapid DNA-Seq Kit, and sequencing was performed on the Illumina MiSeq PE300 platform (Shanghai Majorbio Bio-Pharm Technology Co. Ltd.), producing paired-end 300-bp reads. Data analysis was executed on the Majorbio cloud computing platform, beginning with operational taxonomic unit (OTU) clustering for sequence optimization. The microbial community composition was analyzed and visualized statistically using R software, with taxonomic distribution represented in community bar plots [5]. The microorganisms showing significant differences at the genus level were determined through the Kruskal–Wallis H test, followed by false discovery rate (FDR) adjustment for multiple comparisons. The linear discriminant analysis (LDA) effect size (LEfSe) was employed to determine the significantly enriched taxa (from phylum to genus level) of bacteria across different groups (LDA score > 2, p < 0.05) [28]. The detailed sequence information was presented in Table S4. The sequence quality control process was as follows: initially, the fastp software was employed for quality control of the paired-end raw sequencing reads, followed by sequence assembly using FLASH software. Subsequently, UPARSE software was utilized for Operational Taxonomic Unit (OTU) clustering and chimera removal, based on a 97% similarity threshold of the quality-controlled, assembled sequences. To mitigate the influence of sequencing depth on the subsequent analyses of Alpha and Beta diversity, the sequence count for all samples was normalized to 20,000, while maintaining an average sequence coverage of 99.2% per sample.
2.9 Nontargeted Metabolomics Analysis of Plasma
Protein precipitation was achieved by introducing 400 μL of the extract (acetonitrile: methanol in a 1:1 ratio) to 100 μL of plasma, followed by centrifugation to collect the supernatant for subsequent analysis. The plasma non-targeted metabolomics was analyzed using the ultra-high performance liquid chromatography tandem Fourier transform mass spectrometry (UHPLC-Q Exactive HF-X) system manufactured by Thermo Fisher Scientific. The raw LC-MS data underwent preprocessing using Progenesis QI software (Waters Corporation, Milford, USA). Data refinement involved a three-step filtration process to remove technical artifacts: initially, internal standard signals were eliminated, followed by the exclusion of known artifactual features such as instrument noise, column bleeding, and derivatization reagent residues, and finally, redundancy reduction and peak alignment were conducted. Metabolite identification was accomplished by cross-referencing with three major biochemical databases: HMDB (http://www.hmdb.ca/), Metlin (https://metlin.scripps.edu/), and the Majorbio Database. The Majorbio cloud platform (http://cloud.majorbio.com/) was utilized for data analysis [29]. Differential metabolites were identified by conducting Kruskal–Wallis H test to compare multiple groups. Orthogonal partial least squares discriminant analysis (OPLS-DA) was performed using the R package ropls (Version 1.6.2) for comparisons between CN_LPS versus CN, and FOS_LPS versus CN_LPS. Variable importance in projection (VIP) scores derived from the OPLS-DA model were utilized to identify metabolites that significantly contributed to group separation (VIP > 1, p < 0.05). The screened differential metabolites were further employed to construct the metabolic set, followed by VIP value analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis.
2.10 Statistical Analysis
The data were subjected to analysis using SPSS 20.0 (SPSS Inc., Chicago, IL, USA). The impact of dietary treatment on pathological scores and inflammatory factors was evaluated using Duncan's multiple comparison test. Statistical significance was established at the p < 0.05 level. Mean values accompanied by the pooled standard error of the mean (SEM) were reported, and GraphPad Prism 8.0 was employed for generating graphical representations. Due to the limited number of differentially expressed genes and metabolites identified in multi-group comparisons, FDR correction was not applied for these analyses. Pearson correlation coefficients were utilized for conducting the correlation analysis.
3 Results
3.1 Pathological Assessment of Oviduct Magnum and Expression of Plasma Inflammatory Factors and Immune-Related Genes in Oviduct Magnum
Pathological lesions in the magnum were assessed via HE staining. No signs of inflammation or glandular atrophy were observed in the CN group. In contrast, LPS stimulation induced inflammatory infiltration in the laminae propria of the magnum and marked glandular atrophy in the CN_LPS group, with over 75% of the mucosal lamina propria affected. Through pathological scoring and histological analysis, it was determined that inflammation and atrophy were alleviated after FOS supplementation in the diet. Furthermore, the atrophy area accounted for less than 25% of the total lamina propria (Figure 1A–B). After LPS challenge, plasma IL-6 levels increased significantly, whereas IL-10 levels decreased compared to the CN group (p < 0.05). FOS supplementation restored IL-10 levels significantly compared to the CN_LPS group (p < 0.05) (Figure 1C). As shown in Figure 1D, mRNA expression of TLR2, MYD88, NF-κB, and COX2 was significantly elevated in the magnum of CN_LPS group relative to those in the CN group (p < 0.05). Compared with CN_LPS, the FOS_LPS group exhibited significantly reduced expression of TLR4, TLR2, MYD88, NF-κB, COX2, TNF-α and IL-1β (p < 0.05).
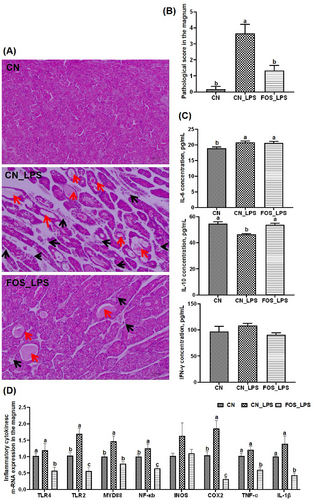
Pathological assessment and inflammatory response of laying hens following LPS challenge supplemented with FOS (n = 6). (A) Histological evaluation of magnum by hematoxylin/eosin staining at 100X magnification. Inflammation is indicated by black arrows, whereas atrophy is indicated by red arrows. (B) Histopathological scoring comparison of the magnum. (C) Analysis of inflammatory cytokines in plasma. (D) Magnum gene expression associated with immunity. The values were presented as means and standard errors, with different letters indicating significant differences among the various groups (p < 0.05). CN, Basal diet + empty capsule; CN_LPS, Basal diet + LPS; FOS_LPS, Basal diet + FOS + LPS.
3.2 Transcriptome Analysis
After quality control and alignment evaluation, RNA-seq data were deemed suitable for downstream analyses (Tables S2 and S3). A volcano plot (Figure 2A) illustrated the distribution of differentially expressed genes (DEGs) (p < 0.05, FC ≥ 2 or ≤ 0.5). In the CN_LPS versus CN comparison, 286 DEGs were upregulated and 104 downregulated. In the FOS_LPS versus CN_LPS comparison, 447 DEGs were identified (51 upregulated, 396 downregulated). Thirty-three overlapping DEGs among the three groups were subjected to KEGG pathway enrichment analysis (Figure 2B). The AMPK signaling pathway emerged as the most significantly enriched, whereas primary bile acid biosynthesis and nonsmall cell lung cancer pathways were also prominently enriched, indicating relevance to inflammatory responses. Cluster analysis revealed that, compared with CN, expression of ABCA9, BIRC5, and MYRF were downregulated in the CN_LPS group but restored in the FOS_LPS group, whereas several inflammation-related genes (e.g., ALPPL, ANGPTL1) followed the opposite trend (Figure 2C).
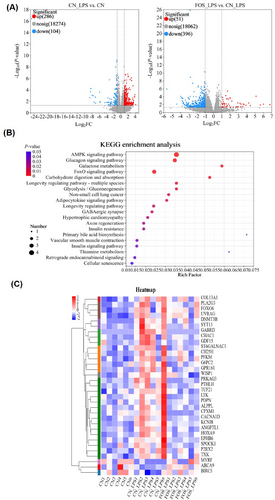
Transcriptomic analysis of the magnum in laying hens following LPS stimulation with FOS supplementation (n = 6). (A) Volcano plot of differentially expressed genes (DEGs) in the magnum of laying hens. Up-regulated genes are indicated by red and down-regulated genes by blue. (B) KEGG enrichment analysis of DEGs. The size of the dot indicates the number of DEGs, and the color of the dot indicates the p-value. (C) Cluster analysis of DEGs. Up-regulated genes are indicated by red and down-regulated genes by blue. CN, Basal diet + empty capsule; CN_LPS, Basal diet + LPS; FOS_LPS, Basal diet + FOS + LPS.
The accuracy of RNA-seq data was validated using quantitative real-time PCR (qPCR) analysis of selected genes TNF-α, MYD88, COX2, TLR2, BIRC5, EDN2, BEST4, and ID4, which showed expression patterns consistent with transcriptomic results (Figure S1).
3.3 Microbiological Analysis
Cecal microbiota composition was analyzed at both phylum and genus levels. Bacteroidota and Firmicutes were dominant phyla across all groups (Figure 3A). The CN_LPS group exhibited higher Bacteroidota abundance but lower Actinobacteriota abundance compared to CN and FOS_LPS groups. Conversely, Firmicutes abundance was higher in the FOS_LPS group (Figure 3A). At the genus level, the abundances of Rikenellaceae_RC9_gut_group, unclassified_o_Bacteroidales, and Alistipes were elevated, whereas the abundances of Lactobacillus, Olsenella, Ruminococcus_torques_group, and Phascolarctobacterium were much lower in CN_LPS as compared to both the CN and FOS_LPS groups. Notably, dietary supplementation with FOS effectively reversed these LPS-induced microbial shifts (Figure 3B). The abundances of Phascolarctobacterium, GCA-900066575, Aeriscardovia, and Rhodococcus were significantly reduced in the CN_LPS compared to both CN and FOS_LPS (p < 0.05), whereas FOS significantly increased Enterococcus abundance (p < 0.05) (Figure 3C). The Lefse multi-level discriminant analysis revealed that Phascolarctobacterium was predominantly enriched in CN, Family_XIII_AD3011_group in CN_LPS, and Anaerofustis, UCG-005, Enterococcus, Anaerofilum, Oscillospira, UCG-009, Aeriscardovia, GCA-900066575, and Rhodococcus in FOS_LPS (Figure 3D–E).
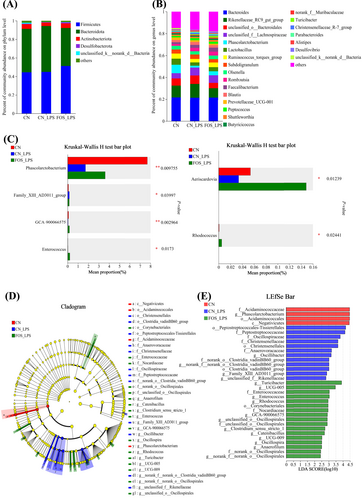
Effect of FOS on the cecal microbiota of LPS-stimulated laying hens (n = 6). Composition analysis of cecal microbes at phylum and genus levels (A and B), with different species represented by distinct colors and their proportions depicted by bar lengths. Differential microorganisms were identified using the Kruskal–Wallis H test (C). Cladogram of the linear discriminant analysis effect size (LEfSe) analysis (D) and linear discriminant analysis (LDA) histogram (E). LDA scores are represented by the length of the histogram, with a log LDA score of greater than 2.0. CN, Basal diet + empty capsule; CN_LPS, Basal diet + LPS; FOS_LPS, Basal diet + FOS + LPS.
3.4 Metabolome Analysis
A total of 20 differential plasma metabolites were identified across groups using the Kruskal–Wallis H test. VIP analysis of differential metabolites highlighted p-cresol glucuronide as the most significant discriminative metabolite between CN_LPS versus CN and FOS_LPS versus CN_LPS. Compared to CN, the CN_LPS group exhibited significantly reduced levels of S-lactoylglutathione, thymol sulfate, Cer(d18:0/16:0), and SM(d18:1/16:0) (Figure 4A). These differential metabolites included 7 organic acids/derivatives, 4 lipids/lipid-like molecules, 3 organoheterocyclic compounds, 3 organic oxygen compounds, and 1 benzenoid (Figure 4B). The KEGG enrichment analysis revealed significant enrichment in several key metabolic pathways, including pyruvate metabolism, galactose metabolism, histidine metabolism, propionate metabolism, nicotinate and nicotinamide metabolism, ascorbate and aldarate metabolism, and pentose and glucuronate interconversion (Figure 4C). Notably, FOS supplementation markedly increased levels of S-lactoylglutathione, propionic acid, thymol sulfate, Cer(d18:0/16:0), and SM(d18:1/16:0) (p < 0.05). Remaining metabolites exhibited opposite trends (Figure S2, Table S5).
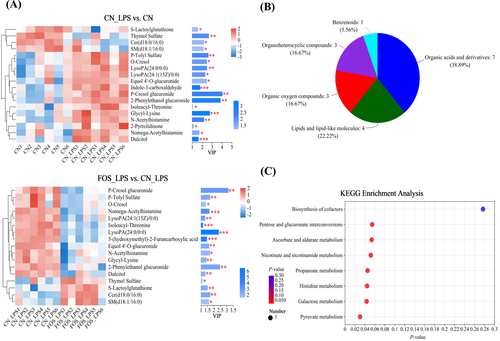
Impact of FOS on plasma metabolites in LPS-stimulated laying hens (n = 6). Analyses of differential plasma metabolites based on VIP scores (A). The color indicates the relative expression of the metabolite in the sample group, and the length of the bar indicates the value of the metabolite's contribution to the difference between the two groups. The larger the value, the larger the difference between the two groups. Analyzing the HMDB database for differential metabolites (B). KEGG signaling pathways enriched by different metabolites. The dot sizes and colors indicate the differential abundances and p-values of the metabolites, respectively (C). CN, Basal diet + empty capsule; CN_LPS, Basal diet + LPS; FOS_LPS, Basal diet + FOS + LPS.
3.5 Correlation Analysis of the Microbiome, Metabolome, and Transcriptome
To explore the integrative mechanism underlying FOS's protective effects against LPS-induced salpingitis, correlation analyses among microbiome, metabolome, and transcriptome were performed. The abundances of Shuttleworthia and Olsenella were positively correlated with propionic acid, which in turn was positively associated with MYRF and negatively with PRKAG3 and GABRD. The Eubacterium_hallii_group showed a positive correlation with S-lactoylglutathione, while Alistipes correlated negatively. In addition, BIRC5 was positively correlated with S-lactoylglutathione and negatively with ST6GALNAC1, PRKAG3, GABRD, PTHLH, CHAC1, and GDF15. The abundance of Phascolarctobacterium exhibited negative correlations with o-cresol, p-tolyl sulfate, and p-cresol glucuronide, while these metabolites were positively associated with CHAC1, PFKM, FOXO6, CH25H, COL13A1, WISP1, PLA2G3, PTHLH, TXK, ST6GALNAC1, PRKAG3, GABRD, KCNJ8, SYT13, SPOCK1, ALPPL, HOXA9, GPR161, ANGPTL1, CACNA1D, TCF21, CPXM1, and G6PC2. Furthermore, p-tolyl sulfate showed a significant negative correlation with BIRC5. The abundance of GCA-900066575 was inversely correlated with dulcitol, while dulcitol also displayed a negative correlation with MYRF. Both dulcitol and 2-phenylethanol glucuronide were positively associated with a suite of inflammation-related genes, including PFKM, ST6GALNAC1, PRKAG3, GABRD, P2RX2, SYT13, ALPPL, ANGPTL1, CACNA1D, G6PC2, and LTK (Figure 5A–B).
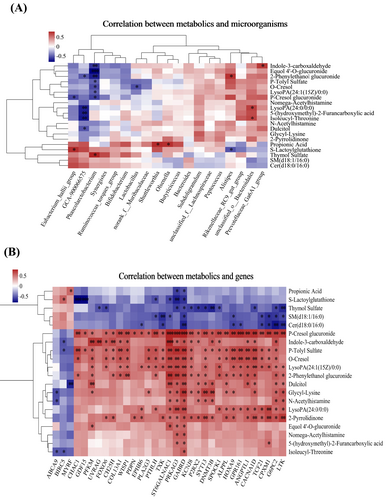
Integrated multi-omics analysis (n = 6). Correlation analysis was conducted between the significant microorganisms in the cecum and the differential metabolites in plasma (A), as well as between the significant differential metabolites in plasma and the differentially expressed genes in the magnum (B). The color red indicates a positive correlation, while the color blue represents a negative correlation. The symbols ***, **, and * denote different levels of significance (p < 0.001, p < 0.01, and p < 0.05, respectively).
4 Discussion
FOS exhibits diverse biological functions and has been utilized in the treatment of various diseases. To examine the impact of FOS on fallopian tube health, this study employed an LPS-induced salpingitis model and assessed the effects of FOS through histopathological evaluation and inflammatory marker profiling. Following LPS stimulation, the magnum exhibited considerable inflammation and atrophy, accompanied by elevated plasma levels of the pro-inflammatory cytokine IL-6 and decreased levels of the anti-inflammatory cytokine IL-10. However, the administration of FOS significantly ameliorated the LPS-induced tissue damage, reduced IL-6 concentrations, and increased IL-10 levels. These findings are consistent with previous studies demonstrating the anti-inflammatory properties of FOS, including its ability to suppress IL-6 and enhance IL-10 expression [30, 31].
LPS-induced inflammation is known to activate the MAPK/NF-κB signaling pathway [26], and our findings showed increased expression of TLR2, MYD88, NF-κB, and COX2 following LPS challenge. Conversely, FOS supplementation downregulated TLR4, TLR2, MYD88, NF-κB, COX2, TNF-α, and IL-1β, suggesting that FOS attenuates inflammation by modulating this pathway. The observed inverse correlation between IL10 and COX2/NF-κB expression supports the hypothesis that FOS enhances IL-10 levels, thereby suppressing pro-inflammatory gene expression [32]. The inhibitory effects of FOS on NF-κB-related genes may be mediated through the production of short-chain fatty acid (SCFA) by gut microbial fermentation. SCFAs are known to activate G protein-coupled receptors (GPRs), inhibit histone deacetylases (HDACs), reduce NF-κB expression, and ultimately decrease the secretion of pro-inflammatory cytokines [31]. Hence, the anti-inflammatory and antiatrophic effects of FOS on the magnum are likely attributed to its suppression of inflammatory signaling cascades.
To further elucidate the mechanisms underlying the anti-inflammatory effects of FOS, we conducted a comprehensive multi-omics analysis encompassing transcriptomics of the magnum, intestinal microbiome profiling, and plasma metabolomics. Transcriptome analysis identified significant changes in gene expression associated with FOS-mediated salpingitis alleviation. KEGG enrichment revealed prominent involvement of the AMPK signaling pathway, including genes such as PRKAG3, PFKM, FOXO6, and G6PC2, all of which are closely related to glycolysis. Previous studies have linked AMPK activation with enhanced glycolysis in cancer and inflammatory contexts [33-36]. The glycolytic pathway not only supplies energy but also generates intermediates that activate the NLRP3 inflammasome, amplifying inflammation [37]. Furthermore, LPS-stimulated AKT signaling promotes glycolysis and pro-inflammatory cytokine production in macrophages [38]. Our data indicate that FOS supplementation downregulates glycolysis-associated genes, potentially reducing the energy expenditure required for inflammatory cytokine production and limiting their expression. In addition, our transcriptomic analysis linked inflammation to the nonsmall cell lung cancer pathway via LTK. LPS-induced upregulation of LTK activated the phosphocarnosine 3 kinase (PI3K) pathway, disrupted endoplasmic reticulum transport, and promoted autoreactive B cell activation [39]. FOS supplementation downregulated LTK, thereby suppressing PI3K signaling and downstream of NF-κB activation. Several DEGs in this study—including P2RX2, SPOCK1, PDPN, and TXK—are known regulators of the NF-κB pathway [40-43]. Their upregulation following LPS exposure likely amplified inflammation, while FOS reversed their expression, mitigating inflammatory responses. Other inflammation-related genes (CHAC1, TCF21, PTHLH, GDF15, CH25H) were also upregulated by LPS and downregulated by FOS. These genes contribute to oxidative stress, apoptosis, and immune dysregulation [44-49]. Notably, FOS restored MYRF and BIRC5 expression—genes involved in neural development and cellular proliferation, respectively—suggesting that FOS supported tissue repair and recovery from inflammatory damage [50-52]. The observed transcriptomic changes indicate that FOS supplementation mitigated inflammation by inhibiting glycolysis and inflammatory signaling pathways while promoting neurodevelopment and cellular proliferation, ultimately improving magnum tissue health. These effects are likely mediated through modulation of the gut microbiome [14, 31].
Gut microbiota plays a crucial role in poultry health by supporting nutrient absorption, immune modulation, and host defense [53, 54]. The dominant phyla observed in this study were Bacteroidota and Firmicutes, which aligns with previous research studies finding [54]. In this study, FOS supplementation increased the relative abundance of Firmicutes, a phylum associated with improved nutrient utilization, anti-inflammatory status, and suppression of pathogens such as Clostridium perfringens [55-57]. At the genus level, LPS increased the abundance of Rikenellaceae_RC9_gut_group and Alistipes, while reducing the abundance of Lactobacillus, Olsenella, Ruminococcus_torques_group, and Phascolarctobacterium—alterations linked to dysbiosis and inflammation [58]. Alistipes has been associated with various pathological conditions and exhibits a positive correlation with the pro-inflammatory cytokine IFN-γ. In contrast, Olsenella shows an inverse association with IFN-γ [59]. This finding helps to explain the increased plasma concentrations of IFN-γ after LPS stimulation. FOS supplementation restored beneficial genera such as Phascolarctobacterium, GCA-900066575, Aeriscardovia, Enterococcus, and Rhodococcus. Phascolarctobacterium produces propionic acid, which inhibits lipogenesis and inflammation [28], whereas Enterococcus improves egg weight and gut health [60]. Moreover, Phascolarctobacterium has been found to hinder the growth of Clostridium difficile through succinate metabolism [61]. Additionally, it reduces levels of LPS-binding protein and C-reactive protein, thereby attenuating inflammation and preserving the integrity of the intestinal mucosal barrier [28]. Thus, dietary supplementation with FOS increases the abundance of Phascolarctobacterium and decreases the abundance of Alistipes, potentially due to a reduction in pro-inflammatory cytokines and C-reactive protein, thereby alleviating salpingitis. Furthermore, GCA-900066575 and Aeriscardovia support intestinal pH and nutrient absorption, and Rhodococcus contributes to detoxification processes [60, 62, 63]. These microbial changes likely reinforced intestinal barrier integrity, suppressed inflammatory cytokines, and improved overall gut health, thereby alleviating salpingitis.
To date, several studies have provided evidence for the impact of intestinal microbiota on the well-being of distant organs through blood metabolites, such as the gut-liver axis [64] and gut microbiota-ovary axis [20]. To explore the systemic impact of gut microbial modulation, we conducted nontargeted plasma metabolomics. LPS challenge elevated toxic metabolites such as 2-phenylethanol glucuronide, dulcitol, and N-acetylhistamine, while reducing beneficial compounds such as propionic acid and S-lactoylglutathione. FOS supplementation effectively reversed these trends. Elevated 2-phenylethanol glucuronide and dulcitol are associated with mitochondrial dysfunction and oxidative stress [65-67], while S-lactoylglutathione enhances detoxification and immune modulation likely via endogenous production of glutathione [68-70]. The increase in propionic acid following FOS supplementation supports previous findings linking SCFAs to inflammation suppression through MAPK and NF-κB inhibition [71-73]. Moreover, FOS also reduced levels of harmful phenolic metabolites such as p-tolyl sulfate, o-cresol, and p-cresol glucuronide, which are known to promote reactive oxygen species (ROS) and NF-κB activation [74-76]. The differential metabolites identified in this study were most prominently enriched in pyruvate metabolism and galactose metabolism. Previous studies have demonstrated that inhibition of pyruvate oxidation reprograms pyruvate metabolism, thereby enhancing NLRP3 inflammasome activity [77]. In contrast, our findings revealed that FOS supplementation promoted pyruvate metabolism, which may alleviate inflammation by suppressing NLRP3 inflammasome activation. Similarly, increased galactose metabolism has been shown to induce galactose accumulation, exacerbating oxidative stress and gut dysbiosis [78]. Notably, FOS supplementation in this study suppressed galactose metabolism, thereby effectively mitigating oxidative stress and restoring gut microbial homeostasis. These results collectively suggest that FOS enhances host metabolism, detoxification, and immune homeostasis.
Finally, correlation analyses among differential metabolites, microbiota, and gene expression further clarified the regulatory mechanisms of FOS. Increases in beneficial genera such as Shuttleworthia, Olsenella, Eubacterium_hallii_group, Phascolarctobacterium, and GCA—900066575, alongside a reduction in Alistipes were strongly associated with anti-inflammatory effects and improved barrier function [28, 53, 59, 62, 79]. This microbial rebalancing likely reduced systemic toxin levels, increased SCFA availability, and suppressed key inflammatory pathways, thereby improving magnum tissue health.
Our study demonstrates that dietary FOS supplementation can mitigate LPS-induced salpingitis through modulation of cecal microbiota, metabolic pathway and host gene expression. Specifically, FOS suppressed glycolysis- and NF-κB-related inflammatory gene expression while promoting neurodevelopment and cellular repair genes. These effects were mediated through favorable alterations in gut microbiota composition and plasma metabolite profiles. However, there are limitations to this study. First, the sample size was relatively small, and the mechanisms inferred form multi-omics analyses required further experimental validation. Future studies will focus on the roles of specific microbial taxa and metabolites identified here. In addition, genetic and microbiome differences among breeds or species may limit the generalizability of these findings. Further research is needed to assess the efficacy of FOS in other animal models or species. Given that bacterial infections are a common cause of salpingitis in both poultry and humans, our findings provide important insights into the potential allocation of FOS in managing salpingitis-related infertility.
5 Conclusions
In summary, the protective effects of FOS against salpingitis are closely linked to its ability to modulate the cecal microbiota and systemic metabolism. Specifically, FOS supplementation altered the abundance of key bacterial genera, including increased levels of Phascolarctobacterium, Eubacterium_hallii_group, Olsenella, Shuttleworthia, GCA-900066575, while reducing Alistipes. In parallel, FOS reduced the plasma concentrations of inflammatory and potentially toxic metabolites such as p-cresol glucuronide, dulcitol, and 2-phenylethanol glucuronide, while enhancing beneficial metabolites such as propionic acid and S-lactoylglutathione. These microbial and metabolic shifts contributed to the downregulation of genes associated with glycolysis, NF-κB signaling, and inflammatory response, and upregulation of genes involved in neurodevelopment and cell proliferation (Figure 6). Collectively, these molecular and physiological changes culminated in the attenuation of LPS-induced salpingitis, underscoring the therapeutic potential of FOS in preventing reproductive tract inflammation. This study provides important insights into the multifaceted mechanisms by which FOS exerts its protective effects and supports its application as a dietary intervention for the prevention of salpingitis.
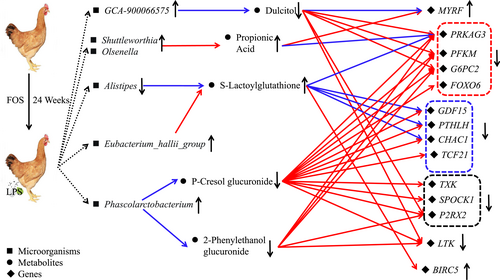
Schematic representation of the regulatory network of FOS for salpingitis prevention. The color red indicates a positive correlation, whereas the color blue represents a negative correlation.
Author Contributions
Dan Song: writing – review and editing, writing – original draft, data curation, investigation, methodology, project administration, visualization, funding acquisition. Tao Duan: data curation. Rong Li: data curation. Xiaoqiu Wang: writing – review and editing. Fengdong Zhang: writing – review and editing. Jia Feng: writing – review and editing. Lin Qiao: writing – review and editing. Junlin Cheng: resources, Lixian Chen: resources. Aike Li: project administration. Yuna Min: writing – review and editing, funding acquisition, supervision, project administration. Weiwei Wang: writing – review and editing, supervision, project administration.
Acknowledgments
This study was supported by the Research Fund for National Non-profit Institutions (Grant ZX2517), and the China Agriculture Research System of MOF and MARA (CARS-40-S20).
Ethics Statement
All experimental procedures were approved by the Animal Ethics Committee Guidelines (registration number: 2021L12) of the Academy of National Food and Strategic Reserves Administration (ANFSRA, Beijing, China), and conducted in accordance with its guidelines.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The datasets utilized or examined in this study can be obtained upon reasonable request from the corresponding author.




