Dietary Baicalin Supplementation Can Enhance the Growth Performance of Weaned Piglets and Maintain the Intestinal Barrier Integrity
Funding: This work was supported by the Key Research and Development Program of Shaanxi Province (2023-YBNY-109), the National Natural Science Foundation (32372845), Qin Chuang Yuan Innovation and Entrepreneurship Talent Project (QCYRCXM-2022-212), Shaanxi Livestock and Poultry Breeding Double-chain Fusion Key Project (2022GD-TSLD-46), China Agriculture Research System of MOF and MARA (CARS-35-PIG).
Yuhui Gao, Jie Liu, and Rui Yang contributed equally to this work.
ABSTRACT
This study aimed to elucidate the promoting effect of baicalin (BAI) on the growth performance of nonpathological piglets and its potential mechanism of improving intestinal barrier function. In this experiment, 240 21-day-old weaned piglets were randomly divided into four treatment groups, consisting of a control group (CON) and groups supplemented with 0.05%, 0.10%, and 0.15% BAI. Compared with the CON group, BAI significantly decreased the ratio of average daily feed intake to average daily gain (F/G ratio) on days 0–7 and 7–14 (p < 0.05) and reduced the diarrhea rate in piglets. Additionally, the 0.10% and 0.15% BAI groups had significantly higher villus height (VH; p < 0.01), and the 0.10% BAI group had a significantly higher Villus height to crypt depth ratio (VCR; p < 0.05), indicating an enhanced intestinal absorption capacity. BAI supplementation significantly increased the expression of intestinal tight junction proteins and antioxidant enzymes, the number of goblet cells, and mucin2 (MUC2) expression (p < 0.05); reduced the expression of pro-inflammatory factors; and improved the intestinal microbiota structure. Specifically, adding 0.10% BAI significantly increased the abundance of beneficial microorganisms such as Eubacterium and Blautia (p < 0.05), while reducing the composition of harmful microorganisms such as Proteobacteria and Spirochetes. Metabolomic results revealed that BAI supplementation significantly increased the concentrations of beneficial metabolites for gut health, such as taurine and rosmarinic acid (p < 0.05). In conclusion, this experiment elucidated the potential ability and intrinsic mechanism of BAI in protecting the intestinal health of piglets and ameliorating the problem of weaning stress.
Abbreviations
-
- ADFI
-
- average daily feed intake
-
- ADG
-
- average daily gain
-
- ALB
-
- albumin
-
- AOD
-
- average optical density
-
- BAI
-
- baicalin
-
- BW
-
- body weight (used for live weight)
-
- CAT
-
- catalase
-
- CD
-
- crypt depth
-
- F/G
-
- average daily feed intake to average daily gain ratio
-
- GCN
-
- goblet cell numbers
-
- HDL-C
-
- high-density lipoprotein cholesterol
-
- IgA
-
- immunoglobulin A
-
- IL
-
- interleukin
-
- Keap1
-
- Kelch-like ECH-associated protein 1
-
- LDA
-
- linear discriminant analysis
-
- LDL-C
-
- low-density lipoprotein cholesterol
-
- MUC
-
- mucin
-
- NMDS
-
- nonmetric multidimensional scaling
-
- Nrf2
-
- nuclear factor E2-related factor 2
-
- OPLS-DA
-
- orthogonal partial least squares discriminant analysis
-
- ROS
-
- reactive oxygen species
-
- SEM
-
- standard error of the mean
-
- SOD
-
- superoxide dismutase
-
- TLR4
-
- toll-like receptor 4
-
- TNF-α
-
- tumor necrosis factor-α
-
- TP
-
- total protein
-
- TRAF6
-
- TNF receptor associated factor 6
-
- VCR
-
- villus height to crypt depth ratio
-
- VH
-
- villus height
-
- VIP
-
- variable importance projection
1 Introduction
After weaning, piglets can suffer from oxidative stress due to changes in nutritional sources, living environment, and exposure to pathogens, which can affect piglet health [1]. Research has demonstrated that the piglet intestine is highly susceptible to oxidative stress, which can harm the intestinal barrier function and alter the gut microbiota [2]. The gut microbiota and intestinal barrier are closely linked to maintaining the balance between the host's intestine and other organs, and disturbances in the gut microbiota could lead to intestinal inflammation, diarrhea, and even death [3]. Previous studies have found that antibiotics used as feed additives could effectively alleviate weaning stress in piglets [4]. However, the overuse of antibiotics in large-scale pig farms is detrimental to human health and has led many countries to ban the addition of antibiotics to feed [5].
Plant extracts have a long history as antimicrobial agents, containing specific bioactive components with good antibacterial, antiviral, antioxidant, and anti-inflammatory effects [6, 7]. This characteristic is particularly significant during the vulnerable period following the weaning of piglets, encountering various challenges, including underdeveloped intestinal barrier function, oxidative stress, dysbiosis, and a heightened incidence of diarrhea. In response to these challenges, the use of antibiotics has become increasingly prevalent, resulting in the emergence of antibiotic resistance. This situation necessitates innovative solutions to address these issues, such as the development of plant extracts. Existing studies have demonstrated that various plant extracts, including oleum cinnamomi, resveratrol, and curcumin, play a significant role in enhancing intestinal health in piglets [8-10]. Therefore, under the backdrop of an antibiotic-free diet, the development of plant extracts that can enhance the gut health of piglets, alleviate weaning stress, and lower the prevalence of diarrhea is of paramount importance.
BAI is a flavonoid compound derived from Scutellaria baicalensis, which has garnered interest as an alternative to antibiotics due to its capacity to target pathogenic microorganisms and promote intestinal health. Beyond its well-documented anti-inflammatory, antioxidant, and antimicrobial properties [11], BAI directly addresses key postweaning challenges by stabilizing gut microbiota and repairing intestinal barrier integrity. Studies have shown that BAI improves gastrointestinal function, treats intestinal damage in food-allergic rats, and can potentially treat diarrhea and gastrointestinal toxicity [12, 13]. Additionally, BAI exerts anti-inflammatory and immunomodulatory effects by inhibiting the NF-κB signaling axis [14]. Previous research has shown that adding BAI to the diet could regulate the intestinal microbiota of mice, improve immunity, promote feed intake, and consequently enhance their growth performance [15]. Furthermore, BAI could potentially protect piglets against pathogenic infections, such as Haemophilus parasuis, by mitigating the release of pro-inflammatory factors [16]. Recent studies have revealed that supplementing BAI in the postweaning piglet diet improved intestinal function, alleviated stress-induced intestinal inflammation, and reduced oxidative damage [17]. These findings presented above suggest that BAI, as a plant extract, possesses a variety of effects, and it holds considerable potential in mitigating intestinal damage resulting from weaning.
The intestine is the primary site of digestion and absorption of nutrients in piglets, playing a crucial role in weaning stress [18]. Under certain conditions, weaning stress might disrupt the intestinal barrier of piglets. The intestine is protected by four barriers: the physical barrier, composed of epithelial cells; the chemical barrier, maintained by goblet and Paneth cells; the immune barrier, regulated by inflammatory factors and cytokines; and the microbial barrier, maintained by symbiotic bacteria [19-22]. Recent studies indicated that BAI has various effects, including promoting intestinal motility and digestive enzyme production in weaned piglets and protecting the intestinal barrier integrity of weaned piglets [16, 23-25]. However, despite these advances, it remains unclear whether BAI can be utilized as a feed additive to enhance the growth performance of weaned piglets under normal physiological conditions, which is essential for meeting the demands of the antibiotic-free farming industry. Furthermore, while the broad-spectrum effects of BAI in protecting piglet gut health have been demonstrated, the specific mechanisms targeting the four types of intestinal barriers still require clarification, limiting its precise application.
Building on this evidence, our study uniquely addresses these dual limitations. In this study, we designed a novel experimental procedure, adding various BAI concentrations to the diets of 21-day-old weaned piglets for 28 consecutive days. Through this study, we explored the potential of BAI in safeguarding the intestinal health of nonpathological piglets, addressing the challenge of weaning stress and elucidating its underlying mechanisms.
2 Materials and Methods
2.1 Experimental Design and Animal Feeding
-
ADG = (body weight at the end of the experiment – body weight at the beginning of the experiment)/(number of piglets × number of days in the experiment).
-
ADFI = total feed intake/(number of piglets × number of days in the experiment).
-
F/G = ADFI/ADG.
| Items | Content |
|---|---|
| Ingredients | |
| Corn | 53.37 |
| Extruded soybean | 10.00 |
| Soybean meal | 16.00 |
| Whey powder | 12.00 |
| Fish meal | 5.00 |
| Limestone | 1.00 |
| CaHPO4 | 0.78 |
| Soybean oil | 0.72 |
| NaCl | 0.30 |
| L-lysine·HCl | 0.39 |
| DL-methionine | 0.16 |
| L-tryptophan | 0.03 |
| L-threonine | 0.15 |
| Premixa | 0.10 |
| Total | 100.00 |
| Nutrient levelsb | |
| DE/(MJ/kg) | 14.47 |
| Crude protein | 18.47 |
| Total P | 0.54 |
| Total Ca | 0.71 |
| STTD phosphorus | 0.48 |
| SID Lys | 1.53 |
| SID Met | 0.49 |
| SID Trp | 0.27 |
| SID Thr | 0.92 |
- a The premix provided the following per kg of the diet: VA 10 500 IU, VD 3300 IU, VE 22.5 IU, VK 3 mg, VB2 7.5 mg, VB1 0.03 mg, K (as potassium iodide) 19 mg, Fe (as K as potassium iodide) 19 mg, Fe (as ferrous sulfate) 333 mg, Se (as sodium selenite) 6 mg, Cu (as cupric chloride) 10 mg, Mn (as manganese sulfate) 129 mg, Zn (as zinc oxide) 400 mg, choline chloride 500 mg, biotin chloride 500 mg, biotin 0.12 mg, folic acid 1.5 mg, niacin 30 mg, and calcium pantothenate 30 mg.
- b Crude protein, total P, and total Ca were measured values, whereas the rest were calculated values.
The BAI used in the experiment was in powder form with a purity of 85% and was purchased from Xi’an Ruilin Biotechnology Co.
2.2 Sample Collection
On the 28th day of the feed trial, six piglets with similar initial body weights were selected from each group. Blood samples were collected and stored in liquid nitrogen. Subsequently, they were transferred to the university’s biology platform, where an automatic biochemical analyzer (BIOBASEBK-400, Shandong Boke Biological Industry Co. Ltd., Shandong, China) was used to assess the levels of immunoglobulin A (IgA), total protein (TP), low-density lipoprotein cholesterol (LDL-C), superoxide dismutase (SOD), and other biochemical indicators. All piglets were humanely slaughtered after being electrically stunned at a commercial slaughterhouse. The duodenum and the colon were removed; 2–3 cm of intestinal tissue was cut with sterile surgical scissors, washed in physiological saline, and fixed in 4% paraformaldehyde solution for subsequent research; and 2–3 cm of cecal tissue was cut open with a sterile surgical knife, washed clean in physiological saline, cut into pieces of the size of yellow beans with sterile surgical scissors, rapidly frozen in liquid nitrogen, placed in centrifuge tubes, and thereafter stored in liquid nitrogen before being transferred to a −80°C freezer for later experiments. 1.0 to 1.5 mL of cecal contents were collected into sterile enzyme-free centrifuge tubes, sealed with parafilm, flash-frozen in liquid nitrogen, and stored at −80°C for subsequent 16S rRNA and metabolomics analyses.
2.3 Hematoxylin and Eosin (H&E) Staining
Fixed duodenal samples were obtained from a 4% formaldehyde solution and underwent gradient ethanol dehydration, xylene clearing, paraffin embedding, and sectioning. Following dewaxing and hydration of the sections, they were stained with Harris hematoxylin; differentiated in hydrochloric acid ethanol; blued in running water; and subsequently subjected to eosin staining, gradient ethanol dehydration, xylene clearing, and neutral resin mounting. The images were obtained using a scanner (Pannoramic MIDI 3DHISTECH, Tanger Electronics Co. Ltd., Jinan, Shandong, China), which were observed using the image processing software ImageJ. The villus height (VH), crypt depth (CD), and duodenal VH-to-CD ratio (VCR) were measured.
2.4 Periodic Acid-Schiff (PAS) Staining
Colon samples fixed in 4% paraformaldehyde and embedded in paraffin were sectioned, deparaffinized, hydrated, oxidized with 1% periodic acid for 5–10 min, rinsed with distilled water, immersed in the Schiff (Sigma–Aldrich, #S5133) reagent for 15–30 min in the dark, rinsed with distilled water to remove unbound reagent, counterstained with Harris hematoxylin for 1–2 min, blued in running water, dehydrated with ethanol gradient, cleared with xylene, mounted with neutral balsam, scanned (Pannoramic MIDI 3D Histech, Tanger Electronics Co. Ltd., Jinan, Shandong, China), and observed using the image processing software ImageJ. The number of goblet cells in the colon samples was estimated.
2.5 Immunohistochemistry
Colon tissues were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned for immunohistochemistry (IHC). Colon sections were incubated with a rabbit anti-MUC2 primary antibody overnight at 4°C, followed by an HRP-conjugated goat anti-rabbit secondary antibody for 1 h at room temperature (see Supporting Information S1: Table S1 for antibody information). DAB substrate was applied for 5 min, and sections were counterstained with hematoxylin. The sections were scanned with a Pannoramic MIDI 3D scanner (3D Histech, Tanger Electronics Co. Ltd., Jinan, China) and analyzed using ImageJ software (National Institutes of Health, USA).
2.6 Western Blot
Tissue proteins were initially extracted using the RIPA lysis buffer (Yeson, Shanghai, China), which consisted of 50 mM Tris (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, and a cocktail of protease and phosphatase inhibitors, including sodium orthovanadate, sodium fluoride, EDTA, and leupeptin. A portion of the cecal samples was lysed in the buffer, followed by homogenization and low-temperature centrifugation at 12,000 rpm for 15 min. Proteins were subjected to separation using 10% polyacrylamide gel electrophoresis and performed gel electrophoresis at a voltage of 120 V, followed by transfer onto polyvinylidene difluoride membranes (Millipore, IPVH00010, Billerica, MA, USA). After blocking, the membranes were incubated with the primary antibody overnight at 4°C (see Supporting Information S1: Table S1 for antibody information). The secondary antibody was then incubated at room temperature for 1 h (Bioss, Beijing, China). The bands were then developed using the iBright CL1500 Imaging System (Thermo Fisher Scientific, Massachusetts, USA). Bands were densitometrically measured using ImageJ software, and relative protein expression levels were normalized to β-actin.
2.7 Quantitative Real-Time PCR Analysis
Total RNA was extracted from cecum samples utilizing the AG RNAex Pro RNA Reagent (Accurate Biotechnology, Changsha, China) [26]. The concentration and quality of the RNA were assessed using a DeNovix spectrophotometer (Thermo Fisher Scientific, DeNovix, Waltham, MA, USA). Reverse transcription was performed using the HiScript III RT SuperMix for qPCR (Vazyme, R323-01, China) with 1000 ng of total RNA. Quantitative real-time PCR was performed using 2 × SYBR Green qPCR Master Mix (Vazyme, Q311-02, China) with a StepOnePlus Real-Time PCR System (ABI, USA). All qRT-PCRs were performed in triplicate, and the final volume of the reaction was 10 μL. Relative mRNA levels were calculated by the 2−ΔΔCt method [27]. Primer sequences are listed in Table S2 of Supporting Information S1.
2.8 16S rRNA Sequencing
The OMEGA Mag-Bind Soil DNA Kit (Omega, USA) was employed to isolate and purify total DNA following the manufacturer's protocol. The quality of DNA was verified through agarose gel electrophoresis. The primers 338F (5′- ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) were used to amplify the V3–V4 regions of 16S rRNA genes by PCR [28]. Equal proportions of the purified products were pooled for sequencing on the Illumina MiSeq platform using the MiSeq Reagent Kit v3. Raw sequencing data were processed with the QIIME2 2019.4 software package [29]. Alpha diversity indices, such as Chao1 and Shannon indexes, were utilized to assess the evenness of colonic microbiota. Nonmetric multidimensional scaling (NMDS) was utilized to assess beta diversity. Additionally, linear discriminant analysis (LDA) was conducted alongside effect size measurements using LEfSe to identify differences in species abundance profiles between the two groups. The correlation analysis was performed using R software (version 4.1.2), considering the nonlinear distribution of microbial relative abundance.
2.9 Liquid Chromatography–Mass Spectrometry (LC–MS) Analysis
LC–MS analysis was conducted by BioNovoGene Co. Ltd. (Suzhou, China). In brief, around 100 mg of cecum content was gathered and placed into a 2-mL centrifuge tube, followed by the addition of 0.6 mL of methanol. After centrifugation at 12,000 g for 10 min at 4°C, the supernatant was filtered through a 0.22-μm membrane for LC–MS analysis. Additionally, a 20-μL sample was reserved for quality control. All samples were subjected to analysis using a Q Exactive Focus mass spectrometer (Thermo Fisher Scientific) and a Vanquish liquid chromatograph (Thermo Fisher Scientific) in both positive and negative ion modes. The raw data acquired from LC-MS underwent analysis using ProteoWizard (v3.0.8789) [30] and were processed with the XCMS (v3.12.0) [31] package in R for feature detection, retention time correction, and alignment. The metabolites were identified based on mass accuracy and MS/MS, and their matches were found in databases such as HMDB (http://www.hmdb.ca), MassBank (http://www.massbank.jp/), KEGG (https://www.genome.jp/kegg/), LipidMaps (http://www.lipidmaps.org), mzCloud (https://www.mzcloud.org), and the metabolite database developed by PANOMIX Biomedical Tech Co. Ltd. (Suzhou, China). Orthogonal partial least squares discriminant analysis (OPLS-DA) was conducted to visualize sample separation. The ropls package (v1.22.0) [32] in R identified distinct metabolites among the groups. Significance was determined based on variable importance projection (VIP) > 1 and p < 0.05 (Student's t-test). Differential metabolites were subjected to pathway analysis using MetaboAnalyst (v6.0) [33]. Spearman correlations were used to explore the relationships between microbiota and metabolite data.
2.10 Statistical Analysis
The results were expressed as mean values with standard error of the mean (SEM). All analyses were conducted using SAS (version 9.4, SAS Institute, USA).
2.10.1 Growth Performance
A mixed linear model with repeated measures was applied to analyze longitudinal data: Yijk = μ + Bi + Tj + Pk + eijk, where Yijk is the dependent variable, μ is the overall mean, Bi is the fixed BAI effect, Tj is the time effect, Pk is the pig effect, and eijk is the random error. Post hoc comparisons were conducted using Tukey's adjustment. Both linear and quadratic contrasts were tested to assess dose-dependent effects. The mixed model included a time effect (Tj) to account for longitudinal changes in growth performance across the 28-day feeding trial.
2.10.2 Western Blot, qPCR, and Biochemical Assays
Based on the experimental design, the significance of differences was assessed using one-way ANOVA analysis, followed by Duncan's multiple range test for further comparisons. p-value of less than 0.05 was considered statistically significant and between 0.05 and 0.10 was considered a trend.
3 Results
3.1 BAI Improved the Growth Performance and Alleviated the Diarrhea Rate in Weaned Piglets
We evaluated the impact of supplementing BAI on growth performance of weaned piglets. The growth performance results are presented in Table 2. Compared to the CON group, piglets in the BAI group with 0.15% concentration showed a significant increase in body weight (BW) at 7 and 28 days (p < 0.05); the average daily gain (ADG) of each BAI group concentration during days 0–7 was significantly higher than the control group The F/G ratio decreased during days 0–7 (p < 0.05). Dietary BAI supplementation significantly reduced the diarrhea rate in weaned piglets (p < 0.05) during days 0–14, 14–28, and 0–28. These results indicated that dietary BAI supplementation significantly enhanced the growth performance of weaned piglets and reduced the incidence of diarrhea.
| Items | Baicalin levels, % | SEM | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.05 | 0.10 | 0.15 | ANOVA | Linear | Quadratic | ||
| BW, kg | ||||||||
| 0 d | 6.78 | 6.75 | 6.74 | 6.72 | 0.104 | 0.978 | 0.671 | 0.914 |
| 7 d | 7.40a | 7.76a,b | 7.78a,b | 7.90b | 0.125 | 0.033 | 0.007 | 0.352 |
| 14 d | 9.15 | 9.67 | 9.29 | 9.43 | 0.156 | 0.115 | 0.510 | 0.220 |
| 28 d | 13.60a | 14.06a,b | 14.26ab | 14.71b | 0.267 | 0.038 | 0.004 | 0.976 |
| 0–7 d | ||||||||
| ADG, kg | 0.09a | 0.14b | 0.15b | 0.17b | 0.008 | < 0.001 | < 0.001 | 0.017 |
| ADFI, kg | 0.15 | 0.16 | 0.16 | 0.18 | 0.017 | 0.308 | 0.099 | 0.538 |
| F/G | 2.19a | 1.30b | 1.09b | 1.11b | 0.147 | < 0.001 | < 0.001 | 0.003 |
| 7-14 d | ||||||||
| ADG, kg | 0.25ab | 0.27b | 0.22a | 0.22a | 0.018 | 0.006 | 0.009 | 0.415 |
| ADFI, kg | 0.27 | 0.28 | 0.31 | 0.30 | 0.075 | 0.985 | 0.727 | 0.919 |
| F/G | 1.23a | 1.08a | 1.47b | 1.44b | 0.074 | < 0.001 | 0.003 | 0.447 |
| 14–28 d | ||||||||
| ADG, kg | 0.32a | 0.31a,b | 0.36a,b | 0.38b | 0.018 | 0.001 | 0.001 | 0.294 |
| ADFI, kg | 0.47 | 0.45 | 0.46 | 0.50 | 0.103 | 0.961 | 0.745 | 0.668 |
| F/G | 1.52 | 1.44 | 1.31 | 1.40 | 0.053 | 0.058 | 0.044 | 0.135 |
| 0–28 d | ||||||||
| ADG, kg | 0.24a | 0.26a,b | 0.27a,b | 0.29b | 0.011 | 0.002 | < 0.001 | 0.936 |
| ADFI, kg | 0.34 | 0.34 | 0.35 | 0.37 | 0.047 | 0.948 | 0.618 | 0.740 |
| F/G | 1.43 | 1.29 | 1.30 | 1.34 | 0.040 | 0.062 | 0.125 | 0.032 |
| Diarrhea rate, % | ||||||||
| 0–14 d | 13.39%b | 13.04%b | 8.21%a | 8.21%a | 1.575 | 0.039 | 0.009 | 0.915 |
| 14–28 d | 6.43%b | 3.04%a | 1.43%a | 1.96%a | 1.163 | 0.002 | < 0.001 | 0.027 |
| 0–28 d | 9.91%b | 8.04%a,b | 4.82%a | 5.09%a | 1.281 | 0.030 | 0.006 | 0.413 |
- Note: All of the values are expressed as the means and pooled SEM, n = 60.
- Abbreviations: ADFI, average daily feed intake; ADG, average daily gain; BW, body weight; F/G, average daily feed intake to average daily gain ratio.
- a,b Mean values within a row with different letters differ at p < 0.05.
3.2 BAI Supplementation Improved the Serum Biochemical Indices of Weaning Piglets
The serum biochemical index detection results are shown in Table 3. Compared with the CON group, the 0.15% BAI group had significantly higher IgA (p < 0.05) and insignificantly higher albumin (ALB; p = 0.098), and the 0.05% BAI group had significantly lower LDL-C, indicating a reduced risk of inflammation. The TP levels in the 0.05% and 0.15% BAI treatment groups were significantly higher than those in the CON group (p < 0.05). SOD levels in the serum of BAI-treated groups showed a numerical increase, but the differences were not statistically significant (p = 0.284).
| Items | Baicalin levels, % | SEM | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.05 | 0.10 | 0.15 | ANOVA | Linear | Quadratic | ||
| ALB, g/L | 23.80 | 25.07 | 24.53 | 30.40 | 1.754 | 0.098 | 0.039 | 0.226 |
| AST, U/L | 151.13 | 139.00 | 138.77 | 156.40 | 28.539 | 0.960 | 0.906 | 0.616 |
| CHO, mmol/L | 2.77 | 2.49 | 2.79 | 2.62 | 0.073 | 0.068 | 0.667 | 0.462 |
| GLU-YA, mmol/L | 3.16 | 3.25 | 3.09 | 3.64 | 0.608 | 0.920 | 0.653 | 0.717 |
| HDL-C, mmol/L | 0.69 | 0.76 | 0.78 | 0.79 | 0.083 | 0.803 | 0.390 | 0.712 |
| IgA, mg/dL | 0.80a | 1.00a | 0.77a | 4.27b | 0.518 | 0.003 | 0.002 | 0.013 |
| IgG, mg/dL | 0.90 | 1.00 | 0.93 | 0.87 | 0.062 | 0.512 | 0.567 | 0.218 |
| LDL-C, mmol/L | 1.62b | 1.37a | 1.68b | 1.62b | 0.048 | 0.008 | 0.188 | 0.095 |
| TG, mmol/L | 0.69 | 0.58 | 0.53 | 0.41 | 0.078 | 0.171 | 0.036 | 0.934 |
| UREA, mmol/L | 3.35 | 2.97 | 2.97 | 3.03 | 0.306 | 0.790 | 0.513 | 0.489 |
| TP, g/L | 44.50a | 51.43b | 49.60b | 53.43b | 1.343 | 0.008 | 0.003 | 0.282 |
| CREA-M, μmol/L | 150.50 | 154.67 | 165.87 | 181.83 | 30.151 | 0.883 | 0.458 | 0.850 |
| SOD, U/mL | 33.33 | 51.67 | 39.00 | 41.33 | 6.232 | 0.284 | 0.695 | 0.235 |
- Note: All of the values are expressed as the means and pooled SEM, n = 6.
- a,b Mean values within a row with different letters differ at p < 0.05.
3.3 Dietary Supplementation With BAI Contributed to Improved Intestinal Morphology
As shown in Figure 1A and Table 4, compared to the CON group, the 0.10% and 0.15% BAI groups had significantly higher VH (p < 0.01), and the 0.10% BAI group had significantly higher VCR (p < 0.05). Similar CD was noted in all groups (p > 0.05). These results showed that dietary BAI supplementation promoted improved intestinal morphology, especially higher VCR, indicating an enhanced intestinal absorption capacity.
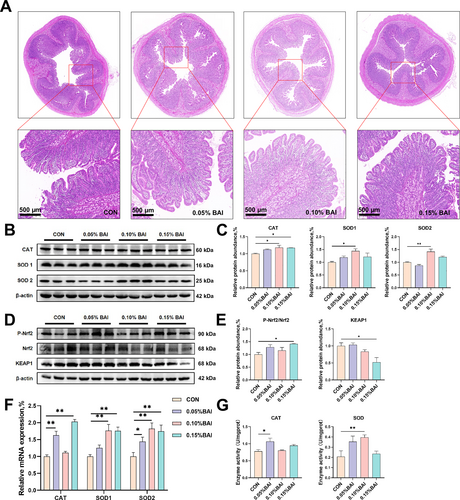
The impact of dietary supplementation with BAI on the intestinal morphology and antioxidant capacity of weaned pigs in normal conditions. (A) The duodenal villus morphology. (B–C) The protein expression of CAT, SOD1, and SOD2. (D–E) The protein expression of P-Nrf2, Nrf2, and KEAP1. (F) The mRNA expression of CAT, SOD1, and SOD2. (G) The enzyme activities of CAT and SOD in cecal tissue. Data are presented as mean ± SEM (n = 6). *p < 0.05; **p < 0.01. 0.05% BAI, basal diet supplemented with the 0.05% baicalin group; 0.10% BAI, basal diet supplemented with the 0.10% baicalin group; 0.15% BAI, basal diet supplemented with the 0.15% baicalin group; CON, corn–soybean-based basal diet group.
| Items | Baicalin levels, % | SEM | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.05 | 0.10 | 0.15 | ANOVA | Linear | Quadratic | ||
| VH | 246.21a | 321.40a,b | 393.27b | 330.81b | 82.16 | < 0.001 | 0.006 | < 0.001 |
| CD | 443.57 | 423.40 | 437.08 | 461.28 | 60.92 | 0.634 | 0.470 | 0.432 |
| VCR | 0.56a | 0.77a,b | 0.94b | 0.73a,b | 0.25 | 0.010 | 0.075 | 0.006 |
| GCN | 800.40a | 1323.40b | 1403.00b | 1330.20b | 358.40 | 0.014 | 0.015 | 0.005 |
| AM | 0.082a | 0.083a | 0.115b | 0.096a | 0.020 | < 0.001 | 0.013 | 0.015 |
- Note: All of the values are expressed as the means and pooled SEM, n = 6.
- Abbreviations: AM, AOD (average optical density) of MUC2; CD, crypt depth; GCN, goblet cell numbers; VCR, villus height to crypt depth ratio; VH, villus height.
- a,b Mean values within a row with different letters differ at p < 0.05.
3.4 BAI Supplementation Strengthened Intestinal Antioxidant Capacity
Next, we investigated the effect of BAI on the antioxidant capacity of the gut. Compared with the CON group, the 0.10% BAI group had significantly higher catalase (CAT), SOD1, and SOD2 in the piglets' cecum (p < 0.05; Figure 1B, C). The level of P-Nrf2/Nrf2 in the 0.15% BAI group was significantly higher than in the CON group (p < 0.05; Figure 1D, E). The relative abundance of the negative regulator Kelch-like ECH-associated protein 1 (Keap1) in the 0.15% BAI group was significantly lower than in the control group (p < 0.05; Figure 1D, E), indicating enhanced antioxidant capacity. Furthermore, RT-qPCR results also showed that the levels of antioxidant-related enzymes in the BAI group were moderately increased (Figure 1F). To investigate the activity of antioxidant enzymes, we used relevant assay kits and found that compared to the CON group, the enzyme activity of CAT was significantly increased in the 0.05% BAI group, and the enzyme activity of SOD was significantly increased in the 0.10% BAI group (p < 0.05; Figure 1G).
3.5 Feeding With BAI Intensified the Expression Level of Tight Junction Proteins
The cecum of the 0.10% BAI group had significantly higher expression levels of claudin-1, claudin-4, and claudin-5 tight junction proteins and mRNA than the CON group (p < 0.05; Figure 2A–C). The mRNA level of ZO-1 in the 0.05% BAI group was significantly higher than in the CON group (p < 0.05; Figure 2C). These above results indicate that the intestinal physical barrier function was improved.
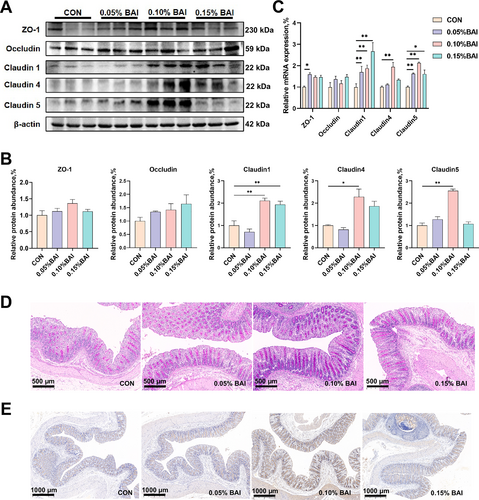
The effect of supplemental baicalin on the physical barrier and chemical barrier functions of weaned piglets. (A–B) The protein expression of ZO-1, occludin, claudin-1, claudin-4, and claudin-5. (C) The mRNA expression of ZO-1, occludin, claudin-1, claudin-4, and claudin-5. (D) The staining of goblet cells in the colon tissue of piglets. (E) The expression level of mucin MUC2. Data are presented as mean ± SEM (n = 6). *p < 0.05; **p < 0.01.
3.6 BAI Supplementation Increased the Number of Intestinal Goblet Cells and Fostered Mucin Generation
As illustrated in Figure 2D and Table 4, PAS staining of colon tissues showed that the number of goblet cells in three BAI groups was all significantly higher than in the CON group (p < 0.05). Furthermore, the MUC2 mRNA levels in the 0.05% and 0.10% BAI groups and the MUC2 protein level in the 0.10% BAI group were significantly higher than in the CON group (p < 0.05; Supporting Information S1: Figure S1 and Figure 2E). These above results suggested that BAI supplementation increased the intestinal mucin content and enhanced the intestinal chemical barrier integrity.
3.7 BAI Inhibited the Activation of Inflammatory Pathways and Regulated the Expression of Inflammatory Factors
Compared with the CON group, significantly lower protein expression levels of toll-like receptor 4 (TLR4) were observed in all BAI groups (p < 0.01; Figure 3A,B). In addition, we also examined the protein expression levels of signaling pathways that regulate inflammation. The protein level of PI3K did not show significant changes (p > 0.05; Figure 3A,C), while the P-AKT/AKT ratio in piglets treated with 0.15% BAI was significantly reduced (p < 0.05; Figure 3A,D), indicating that the inflammatory signaling pathway was inhibited. We also examined the expression of TNF receptor associated factor 6 (TRAF6) and found that there was no significant change (p > 0.05; Figure 3A,E). Compared to the CON group, the phosphorylation level of NF-κB and the downstream tumor necrosis factor-α (TNF-α) expression level in the 0.10% BAI group were significantly decreased (p < 0.05; Figure 3A,F,H,I), and MyD88 protein significantly reduced in the 0.15% BAI group (p < 0.01; Figure 3A,G). All BAI groups showed a downward trend in IL-6 protein expression level (p > 0.05; Figure 3H,J). Meanwhile, the 0.10% BAI group exhibited significantly lower mRNA expression of IL-8, IL-1β, and TNF-α in the piglet cecum tissue (p < 0.05; Figure 3K) and higher expression of IL-4, IL-10, and IL-11 (p < 0.01; Figure 3L). These results suggested that BAI supplementation downregulated the expression of pro-inflammatory factors in nonpathological piglet cecum tissue, inhibited the activation of the TLR4/MyD88/NF-κB signaling pathway, and ultimately decreased the occurrence of intestinal inflammation.
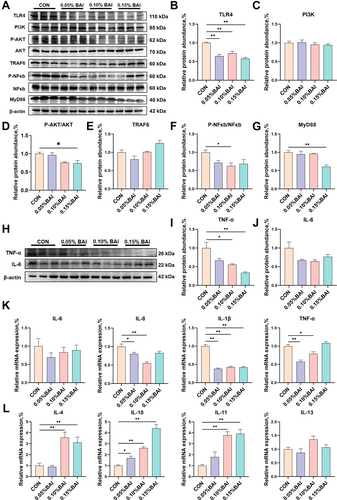
Effects of baicalin on inflammatory pathways and inflammatory factors in the cecum tissue of weaned piglets. (A–G) The expression levels of inflammation-related proteins. (H–J) The protein expression levels of TNF-α and IL-6. (K–L) The mRNA expression of IL-6, IL-8, IL-1β, TNF-α, IL-4, IL-10, IL-11, and IL-13. Data are presented as mean ± SEM (n = 6). *p < 0.05; **p < 0.01.
3.8 BAI Regulated the Intestinal Microbiome and Enhanced the Abundance of Probiotics
The cecal microbiota alpha diversity was evaluated using the Chao1 value and the Shannon index. Both exhibited significant variability (p < 0.05; Figure 4A). Nonmetric multidimensional scaling analysis revealed differences in beta diversity (Figure 4B). Venn diagrams illustrate the shared and unique microbial taxa in the CON and BAI groups (Figure 4C). We examined the relative abundances of microorganisms at the phylum and genus levels to further elucidate the cecal microbiota composition in the CON and BAI groups. Analysis of the top 10 bacterial phylum-level relative abundances showed that Firmicutes and Bacteroidetes were the predominant phyla. The BAI treatment significantly decreased the abundance of Proteobacteria, Spirochetes, and Tenericutes (p < 0.05; Figure 4D,E). At the genus level, we analyzed the compositions of the top 10 genera in the microbiota. The results demonstrated that BAI treatment significantly increased the relative abundance of Eubacterium, Blautia, and Butyricicoccus (p < 0.05; Figure 4F,G), while the change in Coprococcus abundance did not reach statistical significance (Figure 4F,G). We validated the differential taxon abundances using LEfSe analysis. Histograms showing the logarithmic LDA scores > 3 and cladograms are presented in Figure 4H,I. Our study showed that some of the beneficial bacteria, such as Blautia, were positively correlated with the levels of proteins such as tight junction proteins, anti-inflammatory factors, and antioxidants and negatively correlated with the levels of pro-inflammatory factor proteins (Supporting Information S1: Figure S2). The above results suggested that BAI supplementation increased the abundance of probiotics in the intestinal tract of the piglets and improved their intestinal flora.
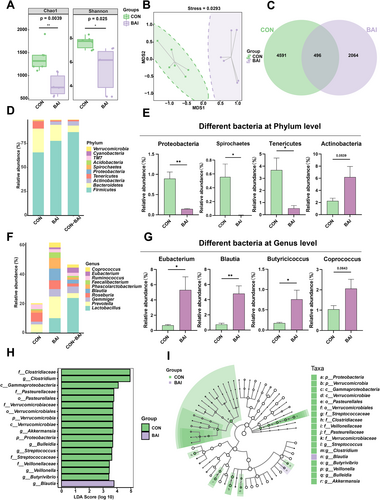
Effects of baicalin on the cecal microbial community of weaned piglets. (A) Alpha diversity analysis of the microbiota. (B) NMDS analysis of the data, with each point representing one sample. (C) Venn diagram of microbial composition. (D) The top 10 phyla are shown. (E) Different bacterial phylum level. (F) The top 10 genuses are shown. (G) Different bacterial genus levels. (H and I) Histogram and cladogram of LDA value distribution. Data are presented as mean ± SEM (n = 6). *p < 0.05; **p < 0.01. BAI, basal diet supplemented with the 0.10% baicalin group; CON, corn–soybean-based basal diet group.
3.9 BAI Regulated Positive Changes in the Piglets' Intestinal Metabolites
The OPLS-DA score plot demonstrated a clear separation between CON and BAI groups (Figure 5A), indicating significant differences in cecal metabolic characteristics between them. Permutation tests confirmed the stability and reliability of the model correlating metabolites with experimental treatments, exhibiting good predictive capability (Figure 5B). We identified 72 secondary differentially expressed metabolites, 21 upregulated and 51 downregulated (VIP > 1, p < 0.05; Figure 5C). The 21 upregulated metabolites included taurine, chenodeoxycholic acid glycine conjugate, rosmarinic acid, and luteolin, whereas the 51 downregulated metabolites included pyroglutamic acid, erucic acid, agmatine, and N-Methyl-D-aspartic acid (p < 0.05; Figure 5D). KEGG pathway analysis revealed that the differential metabolites mainly involved pathways related to alanine, aspartate, glutamate, and histidine metabolism, protein digestion and absorption, and primary bile acid biosynthesis (Figure 5E). Remarkably, the bile acids synthetic pathways crucial in preventing inflammation, exhibited significant enrichment, suggesting an increased ability to counter inflammation. We conducted a Spearman correlation analysis between the top 50 differentially abundant metabolites and differentially abundant bacterial taxa to investigate the relationship between intestinal microbiota and metabolites. Correlation analysis indicated that Blautia was significantly associated with various metabolites such as taurine, rosmarinic acid, and N-methyl-D-aspartic acid and had been identified as a probiotic with anti-inflammatory properties. Notably, Blautia was positively correlated with taurine, which also possesses anti-inflammatory properties, confirming a joint regulatory effect of probiotics and beneficial metabolites on the body (Figure 5F). In addition, data analysis showed that some beneficial metabolites, such as Taurine, were positively correlated with the levels of proteins such as tight junction proteins, anti-inflammatory factors, and antioxidants, and negatively correlated with the levels of pro-inflammatory factor proteins (Supporting Information S1: Figure S3). These results suggest that changes in gut bacterial species and abundance are closely associated with the metabolites therein.
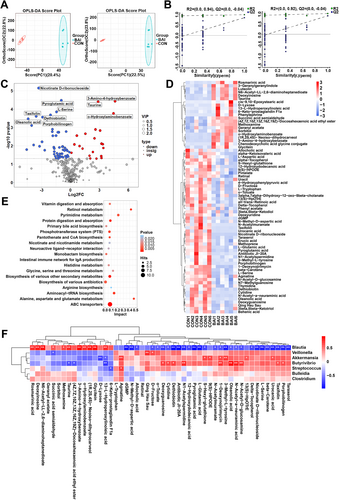
Metabolomic profile analysis of the cecum in weaned piglets. (A) OPLS-DA analysis of the samples. (B) Permutation testing model between metabolites and experimental treatment. (C) Distribution of the metabolites identified in the LDM. (D) Cluster analysis of the differential metabolites. (E) KEGG analysis of the differential metabolites. (F) Correlations between intestinal main differential metabolites and differential microbiota of weaned piglets (n = 6). Blue and red cells represent negative and positive correlations, respectively. *p < 0.05; **p < 0.01. BAI, basal diet supplemented with the 0.10% baicalin group; CON, corn–soybean-based basal diet group.
4 Discussion
Previous research studies have shown that weaning might activate stress pathways in the intestinal of piglets, potentially leading to diarrhea, inflammation, intestinal barrier disorders, and hampered growth performance [18, 34, 35]. Plant extracts are now widely used as antibiotic alternatives for piglets, greatly reducing the damage caused by weaning [36]. BAI is a plant extract with multiple beneficial effects. Studies have shown that BAI can regulate the intestinal microbiota, promote growth performance, and improve intestinal health in mice [15]. In this study, we found that adding BAI to the basic diet of piglets can significantly increase ADG, decrease the F/G ratio, and promote piglet growth, which could be partly attributed to the significant increase in TP (Table 2) that provides the piglets with more energy and proteins [37]. Our results also confirmed that BAI significantly reduced the diarrhea rate in piglets without pathological conditions. Reduced diarrhea rates greatly enhanced piglet growth performance and feed utilization efficiency.
The morphology of the intestine is influenced by various factors, including weaning, environmental changes, and other external factors [38-40]. The functional integrity of intestinal morphology is crucial for intestinal health and the digestion and absorption of nutrients [41], which are typically evaluated through measurements of VH, CD, and VCR [42]. Studies have shown that key digestive enzymes are highly expressed in the small intestinal villi in piglets; therefore, higher intestinal VH and VCR and lower CD indicate better intestinal morphology and digestive function [43]. Studies have shown that premature weaning can cause intestinal damage, including decreased VH and VCR, and increased CD [44-46]. In the present study, we found that BAI supplementation significantly increased the VH and VCR of the duodenum without altering the CD. These results indicate that adding BAI to the diet can alter the intestinal villus morphology of piglets under normal conditions, thereby improving intestinal digestive capacity.
After weaning, piglets are fed a solid powdered diet. This transition often disrupts the physiological balance of intestinal oxidants and antioxidants, leading to excess reactive oxygen species (ROS) that can cause oxidative damage to the piglets’ intestines [47, 48]. Under normal conditions, excess ROS are cleared by antioxidant enzymes such as CAT and SOD to maintain epithelial cell integrity [49]. This study found that the expression levels of CAT, SOD1, and SOD2 significantly increased after adding BAI to the diet, indicating an enhanced antioxidant capacity. The biochemical level of SOD in serum and the enzymatic activity of SOD in tissues are both elevated to a certain extent, which is consistent with our previous experimental results. Furthermore, Nrf2 plays an important role in maintaining redox homeostasis and reducing oxidative stress, while activation of the Nrf2 pathway is negatively regulated by Keap1 [50]. In this study, the antioxidant Nrf2 pathway was activated, with a decrease in the protein expression level of the negative regulator Keap1. Serum albumin can maintain plasma osmotic pressure, possess anti-inflammatory and antioxidant properties, and bind and transport small molecules [51]. Our serum test results showed that BAI supplementation increased piglet serum albumin content and enhanced antioxidant capacity.
The intestinal physical barrier is primarily composed of glycocalyx on the microvilli of intestinal epithelial cells and cell junctions, including tight and adherens junctions, which physically prevent the invasion of microorganisms between them [52]. Tight junctions comprise many proteins, including the transmembrane protein family claudins, the membrane-associated ZO protein family, occludin, and other proteins [53, 54]. Changes in tight junction proteins can affect the paracellular permeability, thereby influencing the resistance to pathogenic microorganisms [55]. As weaning stress affects the physical barrier function in piglets, it leads to reduced mRNA expression of occludin, claudin-1, and ZO-1 [46]. Yang et al. demonstrated that BAI can alleviate endometrial tight junction protein damage induced by LPS in ruminants, thereby treating inflammation [56]. Consistently, we found that BAI increased the expression levels of tight junction proteins, such as ZO-1, claudin-4, claudin-5, and occludin, in the duodenal tissue; improved the paracellular permeability; and enhanced the intestinal physical barrier function.
The cytoplasm of goblet cells, named for their typical goblet-shaped appearance, is filled with mucin granules composed mainly of typical mucin components such as MUC2, FCGBP, and AGR2 [57]. The main function of goblet cells is to synthesize and secrete mucus, a viscous liquid composed of mucin glycoproteins. Mucin glycoproteins can protect the integrity of the host’s intestinal function, mainly achieved through the secretion of MUC2 in the intestine. Studies have shown that MUC2 is an essential intestinal mucosal barrier structural component [58, 59]. It helps prevent infections and strengthens intestinal immunity. Our experimental results indicated that BAI supplementation increased the number of goblet cells in the colon, upregulated the expression level of MUC2 mRNA, stimulated the secretion of MUC2, and enhanced intestinal chemical barrier functions. These findings are consistent with existing literature and our anticipated results [58, 59].
After weaning, piglets often exhibit slow growth and diarrhea, which are related to intestinal inflammation and immune barrier dysfunction [60]. Previous studies have shown that commensal and pathogenic bacteria primarily influence the host’s immune inflammatory responses by activating the TLR4/MyD88/NF-κB signaling pathway in the intestine [61, 62]. Increasing evidence confirms that NF-κB is a significant transcription factor in various inflammatory signal transductions and is regulated by multiple pathways including PI3K/AKT, driving the occurrence of inflammation [63]. Therefore, this study examined the protein levels of the PI3K/AKT/NFκB axis and inflammatory-related factors. We found that the addition of BAI downregulated the expression of pro-inflammatory factors and increased the activity of antioxidant enzymes in the cecum of normal piglets. BAI supplementation also inhibited the activation of the TLR4/MyD88/NF-κB signaling pathway and reduced intestinal inflammation. This was consistent with previous research [14, 62, 63], further confirming the feasibility and necessity of adding BAI as a substitute for antibiotics to piglet diets.
The abundance and diversity of intestinal flora are important determinants of maintaining intestinal health [64]. Our research found that feeding BAI significantly increased gut microbiota diversity. We also observed that BAI supplementation reduced the abundance of Proteobacteria and Spirochaetes, indicating decreased harmful bacteria and healthier gut microbiota [65-67]. Prior research indicated that the Actinobacteria were more abundant in healthy hosts than in unhealthy hosts with diarrhea [68]. Furthermore, we found that feeding BAI significantly increased the abundance of Blautia, Eubacterium, Butyricicoccus, and Coprococcus, indicating an increase in beneficial bacteria in the gut. Previous research has shown that Blautia can produce short-chain fatty acids by utilizing carbohydrates, maintaining colonic mucus function, and exhibiting potential probiotic characteristics in regulating host health [69, 70]. Eubacterium has been reported to increase short-chain fatty acids such as butyrate, propionate, and acetate, which maintain intestinal health in pigs [71, 72]. A previous study indicated that Butyricicoccus can improve feed conversion and reduce the abundance of some potentially important pathogens in the cecum and the ileum, helping prevent necrotic enteritis [73]. Coprococcus is an important producer of butyric acid and serves as a microbial marker for gastrointestinal health. In conclusion, our results demonstrated that feeding BAI can enhance the abundance of beneficial bacteria, reduce harmful bacteria, and improve the gut microbiota composition.
Bile acids, important components of bile, can mix with digestive products in the intestines to facilitate their passage through the intestinal mucosa into the body, thereby enhancing digestive capacity [74]. Moreover, studies have reported that bile acids and their metabolites can regulate intestinal immunity, help maintain intestinal barrier integrity, and decrease the risk of inflammation [75]. In our research, we observed a significant increase in bile acids, vitamins, proteins, and amino acid metabolism processes after adding BAI, with significant enrichment in the primary bile acid synthesis pathway. These results indicate enhanced digestive and anti-inflammatory capabilities. A previous study indicated that the Eubacterium can convert bile acids in the intestine, promoting the synthesis of bile acids [76]. In this study, we found that the abundance of Eubacterium significantly increased after treatment with BAI, which further suggests a potential increase in bile acids. Meanwhile, the BAI groups significantly upregulated 21 metabolites such as taurine, rosmarinic acid, luteolin, and chenodeoxycholic acid glycine conjugate and significantly downregulated 51 metabolites such as agmatine and pyroglutamic acid. This confirms the significant increase in the intermediate metabolites, taurine, and chenodeoxycholic acid glycine conjugate, in the bile acid synthesis process. Both metabolites have been reported to have anti-inflammatory and antioxidant effects [77-79]. In addition, it was reported that rosmarinic acid could regulate the gut microbiota, with anti-inflammatory and antioxidant functions [80], while luteolin can alleviate inflammation in rats with ulcerative colitis and regulate the gut microbiota [81]. Our analysis found a positive association between BAI supplementation and specific microbial changes, thereby influencing specific metabolites. For example, BAI significantly increased the abundance of Blautia, leading to an increase in the metabolic levels of taurine, jointly promoting gut health. This indicates that alterations in specific intestinal microbial communities can impact metabolite levels, suggesting an interaction between the two in regulating the organism's health.
This study elucidates the multidimensional value of BAI within the “One Health” framework: at the level of animal health, it diminishes reliance on farm antibiotics by enhancing intestinal barrier function (including tight junction proteins and mucins), inhibiting inflammatory factors, and reducing the incidence of diarrhea; concerning human health, it specifically targets pathogenic bacteria and enhances the ability to clear viruses, thereby obstructing the dissemination of antibiotic resistance genes and the transmission of zoonotic diseases, together with its plant-derived characteristics and advantages in ecological degradation; it establishes a virtuous cycle of “intestinal health optimization-antibiotic reduction-environmental burden reduction”, offering interdisciplinary solutions for sustainable animal husbandry.
5 Conclusion
This study confirmed the positive effects BAI has on growth performance, intestinal morphology, and intestinal barrier integrity in weaned piglets. Specifically, BAI enhanced the piglet intestinal tissue structure, improved intestinal barrier function, increased antioxidant capacity, and reduced the diarrhea rate in nonpathological piglets. BAI reduced the risk of inflammation, enhanced the intestinal microbial community structure, and stimulated the production of beneficial metabolites. These findings provide an experimental basis for incorporating BAI into piglet diets and offer a new perspective on the BAI's mechanism of action.
Author Contributions
Yuhui Gao: investigation, supervision. Jie Liu: writing – original draft, formal analysis. Rui Yang: writing – original draft, methodology, software. Xizhen Fan: conceptualization, data curation. Cheng Liang: validation, resources. Xin’e Shi: writing – review and editing, funding acquisition. Jianjun Jin: writing – review and editing, funding acquisition, supervision.
Acknowledgments
We thank the Animal Experiment Center of Northwest Agriculture and Forestry University for providing the feeding place, and the Muscle Biology and Pig Genetic Improvement Innovation Team of the College of Animal Science and Technology of Northwest Agriculture and Forestry University for providing the experimental equipment and venues.
Ethics Statement
The study followed the Guidelines for the Care and Use of Laboratory Animals. The Animal Ethics Committee of Northwest A&F University approved all animal experimental procedures in this study (Approval No. IACUC2023-0501, Yangling, China; May 26, 2023).
Conflicts of Interest
The authors declare that they have no competing interests.
Declaration of Generative AI and AI-Assisted Technologies in the Writing Process
During the preparation of this work, the author(s) did not use any AI and AI-assisted technologies.
Open Research
Data Availability Statement
Data are available from the corresponding author upon request.




