Comparison of extraction efficiency and selectivity between low-temperature pressurized microwave-assisted extraction and prolonged maceration
Abstract
Extraction is a key step in studying compounds from plants and other natural sources. The common use of high temperatures in pressurized microwave-assisted extraction (PMAE) makes it unsuitable for the extraction of compounds with low or unknown thermal stability. This study aimed at determining the suitability of low-temperature, short-time PMAE in attaining yields comparable to those of prolonged maceration at room temperature. Additionally, we explored the phytochemical differences of the extracts from both techniques. Maceration at room temperature for 24 hr and PMAE at 40–45°C and 10 bar for 30 min were carried out on 18 samples from 14 plant species at a solvent-to-feeds ratio of 10. The PMAE yields of 16 out of 18 samples were within the proportions of 91–139.2% as compared with the respective extracts from maceration. Varying numbers of nonmatching peaks were noted in MS chromatograms of five extract pairs, indicating selective extraction of some compounds. Low-temperature PMAE can attain reasonable extraction efficiency with the added value of sparing compounds of low thermal stability. The method can also enable the recovery of compounds distinct from those obtained by maceration.
1 INTRODUCTION
The search for bioactive compounds for the treatment of diseases, among other applications, is key to ensure the continuous availability of viable treatment options. A number of approaches can be used in the discovery of new medicines. These include the screening of natural products or chemical libraries, in silico designing, and modification of existing medicines, to mention a few. Extraction is an important step toward obtaining phytochemicals of interest from plant materials. Outcomes of an extraction process are influenced by extraction duration, temperature, pressure, solvent's polarity, and acidity of the extraction medium, among other factors.[1]
Different extraction methods have been reported. Maceration, percolation, infusion, decoction, and Soxhlet extraction are among the most employed techniques. This is mainly due to their less requirement for modern equipment and other infrastructure. However, other modern methods are currently in place. Most of them aim at attaining higher yields, reduced solvent use, and shorter extraction time.[1]
Microwave-assisted extraction (MAE) involves the use of microwaves (300 MHz to 300 GHz) to generate thermal energy through rotation or vibration of dipoles or ionic conduction.[2] However, commercially available laboratory microwave units commonly use a frequency of 2.45 GHz, as other frequencies can interfere with telecommunication and radar systems. In MAE, both heat and mass transfer are directed toward the solvent.[3] The rapid heating generated by MAE causes sudden evaporation of residual water or solvent molecules in plant cells. This results in a buildup of high internal pressure and rupturing of the cells.[3] These events are, thus, in favor of higher rates of desorption, diffusion, and partition of the phytochemicals from the plant matrix into the extracting solvent.[4]
Attaining high recovery rates using conventional methods is a challenging task. Studies have indicated the necessity for longer extraction time and higher temperatures as possible modifications of these methods to boost their efficiency.[5-7] Besides prolonged exposure to atmospheric oxygen, thermal, oxidative, or enzymatic degradations, as well as cross-reactions among the phytochemicals, can occur.[3, 8]
There are two possible equipment modes of carrying out an MAE. In the open mode, the equipment operates at an atmospheric pressure, commonly associated with a refluxing mechanism.[9-11] Modification of domestic microwaves to suit this mode is also a common approach.[8, 12] On the contrary, the closed mode offers the choice of operating at a high pressure. The pressure is built up by the pumping of inert gas into the extraction chamber. Nevertheless, a degree of pressure may be generated by vapor pressure during heating of the extraction mixture.[9, 13]
The use of pressure enables the heating of the solvents above their boiling points. Depending on the phytochemicals of interest, this can result in higher yields and an overall decrease in extraction time.[13, 14] Furthermore, the application of pressure is in line with the working principle of pressurized liquid extraction, whereby, besides enabling heating of the solvent above the boiling point, high pressure improves the permeation of the solvent through the plant matrices, hence favoring the desorption process.[10, 15] The combination of pressure in MAE is also termed as pressurized microwave-assisted extraction (PMAE).[13-16]
Current reports on the use of MAE indicate a broad use of rather high extraction temperatures, mostly in the range of 60–120°C.[4, 5, 17-19] This approach has the benefit of achieving good yields using a few seconds to <10 min. However, it is not suitable for the extraction of heat-sensitive compounds or when the compounds of interest are unknown.
In the current study, we aimed at exploring the usefulness of PMAE when conducted at low temperatures and moderate time duration. Besides evaluating the recovery efficiency of PMAE in comparison to maceration, we also wanted to determine if the obtained extracts differed in phytochemical profiles.
2 RESULTS
A total of 18 plant samples were obtained from 14 plant species (Table 1). The plants were selected on the basis of a parallel study aimed at evaluating the antimicrobial activities of these plants.
| Name | Part(s) studied | Internal accession number |
|---|---|---|
| Acacia melanoxylon R. Br. | Leaves | XXXX-399-E-80 |
| Alpinia purpurata (Vieill.) K. Schum. | Leaves | 2010-88-B-70 |
| Asparagus densiflorus (Kunth) Jessop | Leaves | XXXX-284-P-80 |
| Asparagus officinalis L. | Stem | XXXX-660-G-80 |
| Artocarpus heterophyllus Lam. | Leaves | 1986-42-B-80 |
| Cinnamomum verum J. Presl | Leaves, stem | 2010-90-B-80 |
| Erythrina crista-galli L. | Bark, stem | 1982-348-E-80 |
| Ficus carica L. | Bark, stem | XXXX-220-G-80 |
| Garcinia spicata Hook.f. | Leaves | 1977-306-D-80 |
| Garcinia tinctoria (DC.) W. Wight | Leaves | XXXX-74-B-80 |
| Olea europaea L. | Leaves | XXXX-64-P-20 |
| Paeonia officinalis L. | Leaves | 2013-11-S-10 |
| Prunus sargentii Rehder cv. Rancho | Leaves, bark | 2005-137-M-80 |
| Zingiber officinale Roscoe | Rhizome | Charge 329272 (Kraeuter Mix, Germany) |
2.1 Quantitative comparison of the extract compositions
Of the 18 tested samples, 16 were found to provide MAE yields with the proportions >90% as compared with maceration (Figure 1a). Moreover, 13 samples provided yields within 100 ± 10% of the maceration, with 79% and 139% being the lowest and highest proportions, respectively.
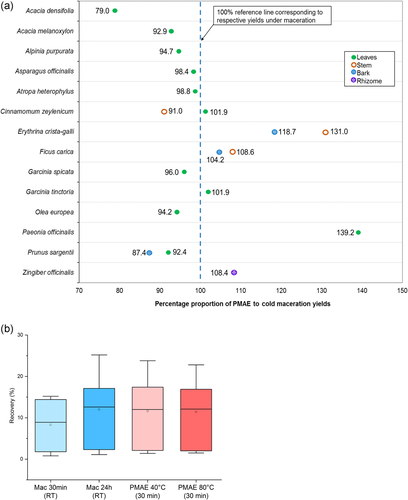
Evaluation based on the plant's part tested revealed that 7 of the 10 leaves samples showed PMAE yields lower (79–99%) than those of maceration. On the contrary, out of the four and three samples from stems and barks, respectively, two of each showed higher PMAE yields (104–131%) than those of maceration (Figure 1a).
Maceration carried out for the same duration as PMAE (30 min) resulted in recoveries lower than those observed under 24-h maceration and 30-min PMAE at 40°C. As displayed by box and whisker plots in Figure 1b, recoveries from PMAE experiments conducted at a higher temperature (80°C) were not superior to those obtained when PMAE was conducted at 40°C.
2.2 Semiquantitative comparison by peak intensities in UV and mass spectrometry (MS) chromatograms
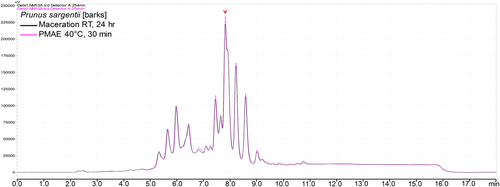
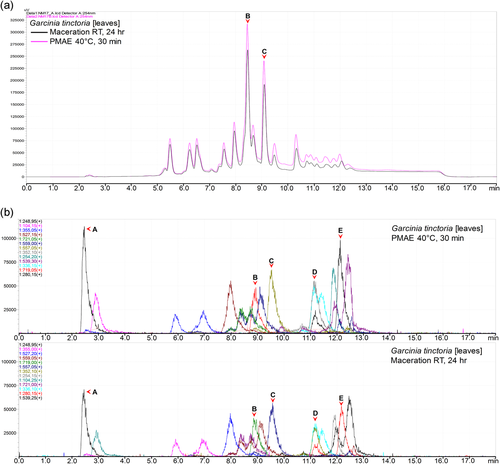
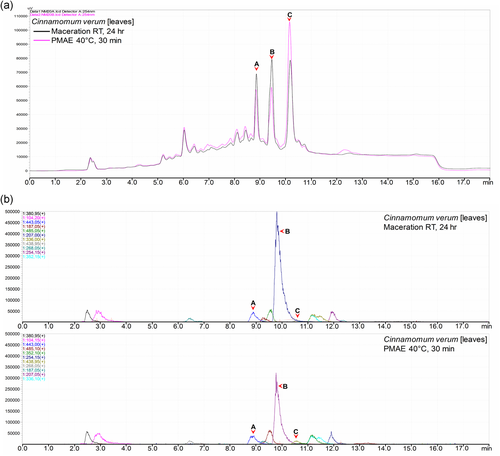
Normalization of the chromatograms was ensured by injecting the same volume and concentration of the sample, as well as maintaining all other chromatographic parameters. Notable differences in peak intensities were observed in all or some of the peaks in corresponding chromatograms of all samples. In 10 out of 18 samples, there were higher intensities of peaks in chromatograms of the extracts prepared from PMAE. The differences in intensities ranged from small (Figure 2) to well notable ones (Figure 3a). A similar pattern was observed in MS base peak chromatograms (BPCs; Figure 3b). Additional figures under this group are shown in Section 2 of the Supporting Information file.
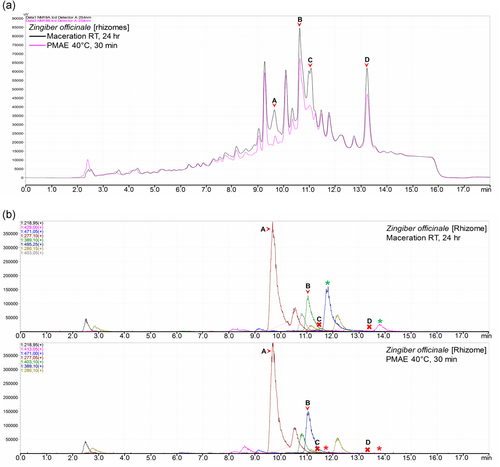
However, in 2 of the 18 samples, the chromatogram of a sample extracted by maceration generally revealed higher intensities of peaks as compared with those of the sample obtained under PMAE (Figure 4a). However, this was not consistent with the intensities observed in the BPCs of Zingiber officinale extracts (Figure 4b) in which peaks A and B had equivalent intensities and peaks C and D were not observable (marked x) due to a possible poor or no ionization, which makes the UV and MS detection hardly comparable.
Furthermore, 6 of the 18 samples showed a combination of the above two scenarios. In these samples, peaks from both extraction methods appeared to have relatively higher intensities at different regions of the UV chromatograms (Figure 5a) and the corresponding BPCs (Figure 5b). Comparable results are given in Section 2 of the Supporting Information.
2.3 Qualitative comparison of the extract compositions
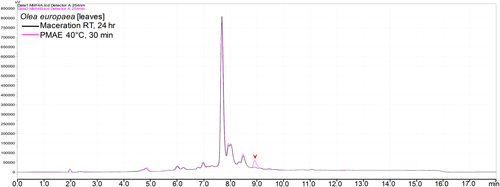
The search for additional/nonmatching peaks on the UV chromatograms indicated that 4 out of the 18 chromatograms contained at least one additional peak within the extracts obtained by both methods. The additional peaks varied in intensities from small, as shown in Figure 6, to notably large, as exemplified in Figure 7a (see also Section 1 of the Supporting Information).
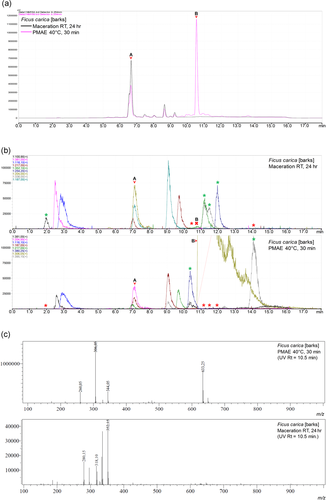
The BPC corresponding to the notable additional peak (B) seen on the UV chromatogram of PMAE extract of Ficus carica barks (Figure 7a) was not found in the corresponding MS BPC of the extract prepared by maceration (Figure 7b). Figure 7c shows the absence of the m/z signal in the maceration extract, corresponding to that observed in the PMAE extract.
However, although we observed nonmatching peaks in the UV chromatograms of Olea europaea leaves (Figure 6), F. carica stem, and Alpinia purpurata leaves extract (Supporting Information), all the peaks observed under MS BPCs for these samples were found to be matching in extracts from both methods (Supporting Information).
Further assessment of the MS BPCs for the presence of new peaks (compounds) in each of the extracts indicated a number of nonmatching peaks in 5 of the 18 studied pairs. We observed peaks that were only present in one type of extract and not the other in Z. officinale (Figure 4b), F. carica bark (Figure 7b), as well as Erythrina crista-galli stem, Garcinia spicata leaves, and Paeonia officinalis leaves extract (Supporting Information). As displayed in Figure 8, the mean and median number of additional BPCs (compounds) found in maceration extracts were slightly higher than those in PMAE extracts.

3 DISCUSSION
Pressurized MAE in a relatively short time (30 min) and at low temperature can achieve recoveries comparable to those in maceration under prolonged duration (24 hr). The two methods are likely to extract different types of compounds, which may affect the magnitude of biological activity under investigation.
Maceration-like yields of crude extracts can still be obtained using relatively lower temperatures during PMAE. Moreover, the quantity of yields obtained is likely to be affected by the nature of the plant matrix.
This is evident based on 16 out of 18 of PMAE extracts showing crude extract with proportions of above 90% in comparison to those of maceration. The majority of PMAE of plant materials is currently in favor of using relatively high temperatures (60–120°C) and short extraction times.[4, 5, 18] Nevertheless, the findings obtained here highlight the possibility of achieving maceration-like outcomes at lower temperatures and moderate extraction time. The lower PMAE yields in the majority of samples of leaves underscore previous reports on the impact of plant matrix on PMAE. This is linked to the presence of residual amounts of moisture in the matrix, which increases MAE efficiency.[3, 20] Moreover, as seen in Figure 1b, higher recoveries can be obtained when maceration is carried out at prolonged durations. The use of high temperature (80°C) in PMAE did not result in superior yields than those obtained at 40°C.
We studied the role of low microwave power and pressure by maintaining other key factors (plant matrix, solvent-to-matrix ratios, and extracting solvent). The observed outcomes are, therefore, independent of these factors. However, the small number of samples from stems and barks may limit the generalizations of the above-drawn conclusion on the role of plant matrices. Moreover, the lower recoveries observed when maceration was carried for 30 min indicate the lack of attainment of extraction equilibrium before this time point. However, the findings have indicated that the use of higher temperatures like 80°C used as a control set in this study does not always guarantee higher recoveries. This is caused by the possible destruction of the plant matrices at higher temperatures, which impair the mass transfer of phytochemicals into the extracting solvent.[19] The need for using high temperatures in PMAE should, therefore, necessarily involve experiments to predetermine the temperature for optimal recoveries.[8, 18]
Low-temperature PMAE can, therefore, be considered as a very useful approach in attaining good yields of phytochemicals. This is more essential when there is no information on the thermostability of the phytochemicals responsible for the activity of interest, because it avoids possible degradations or a cross-reaction of phytochemicals occurring at high temperature and prolonged extraction times.[4, 19]
The use of PMAE can recover higher amounts of all or some of the phytochemicals from the plant matrix. Moreover, a degree of selectivity most likely based on the nature of phytochemicals and their solubility at different temperatures and time conditions is possible.[5, 21] This is demonstrated by the higher intensities of UV and BPC peaks observed in chromatograms of PMAE extracts in 8 out of 14 samples that had no additional UV peaks (Figure 3a,b and Supporting Information). However, as superimposed chromatograms of other 5 out of 18 samples showed a mix in higher intensity peaks, other factors may be in play (Figure 5a,b). Other studies have also indicated differences in selectivity of extracted phytochemicals when MAE was compared with other extraction methods.[4-6]
This can be caused by the nature of phytochemicals present in the matrix and their dependence on temperature and duration of extraction.[3, 22] The magnitudes of peaks' intensities can be directly related to the quantities of respective phytochemicals. This is because, for each sample, the same sample concentration and injection volume were used during high-performance liquid chromatography–mass spectrometry (HPLC–MS) analysis.
When the phytochemicals of interest are known, the application of PMAE at low temperatures is a valid option for a selective increase of their recovery from the plant matrix. This can particularly be beneficial when these compounds are sensitive to high temperatures. However, high heat stability of phytochemicals of interest warrants the use of high temperatures, with further benefits of shorter extraction times and even higher yields.[2, 8, 18]
Differences in profiles of recovered phytochemicals may be imposed by the selected extraction method. Determination of the actual degree of additional compounds is nevertheless subject to the applied detection method.
Our evaluation of HPLC chromatograms under a UV detector showed only 4 (O. europaea leaves, F. carica barks, F. carica stem, and A. purpurata leaves) out of 18 samples to possess new/additional peaks in extracts from at least either one of the extraction methods. Moreover, upon cross-examination of these peaks with the respective mass spectra, only one of them (peak B in Figure 7a) was confidently noted to be additional (Figure 7b,c). The compound corresponding to peak A at the RT of 6.6 min in UV chromatograms had a molecular peak at m/z 815.10 and was likely a caricaflavonol diester A.[23] However, on the basis of literature and library search, we could not ascertain the identity of the compound corresponding to an additional peak B at RT = 10.5.
Varying numbers of additional peaks/compounds were noted in five pairs of extracts from both methods when the MS BPCs were evaluated independent of the UV chromatograms (Figure 8). This enabled us to arrive at a different conclusion in this aspect. Maceration extracts had a slightly higher mean and median numbers of additional BPCs as compared with PMAE (Figure 8). Therefore, these findings show chances of the prospect of recovering completely different types of phytochemicals, based on the method of extraction.[14, 24, 25]
Apart from the detection method, other factors such as method selectivity due to other chromatographic conditions play a role. Through their particular influence on sensitivity and selectivity of the method, these factors are prone to affect a clear observation of additional compounds in generated chromatograms.[7] For example, this study employed a single UV detection wavelength of 254 nm; hence, compounds having chromophores with maximum absorbance at other wavelengths or compounds that lack a chromophore may be missed. These factors may explain the non-UV detection of other compounds, which were detected on the electrospray ionization–mass spectrometry (ESI–MS) detector (Figure 3a,b).
Moreover, poor or lack of ionization of some compounds might have contributed to the observed missing MS BPCs corresponding to some peaks in UV chromatograms (Figure 4a,b). There are low chances that these additional compounds are the products of degradation or cross-reactions among phytochemicals in the crude extracts. This is based on the relatively low temperature and moderate extraction durations used in the study.[3, 18]
In addition to the selection of a suitable extraction method, we underline the need for using more versatile detection methods for evaluation of plant extracts, whenever possible, which may include the use of a tandem arrangement of two or more detectors, if possible. This decreases the necessity of developing specific methods for each plant sample, especially when many samples are to be routinely analyzed.
The common utilization of high temperatures in MAE and PMAE at the expense of losing heat-unstable compounds can be avoided. This study has demonstrated that the use of low temperatures in PMAE has the potential of attaining recovery rates comparable to prolonged maceration and offers a different profile of extracted phytochemicals. Taken together, these can improve the magnitude and range of activities under observation.
In line with previous findings, the nature of plant materials was observed to bear an influence on the quantity of recovered extracts under PMAE as compared with maceration. Among other factors, this is influenced by the ability of the plant material to hold residual moisture even after prolonged drying.
Likewise, fairly higher amounts of individual phytochemicals can be obtained using PMAE as compared with prolonged maceration. This selectivity may be a result of differences in solubility properties of a given phytochemical in relation to extraction temperature and time. Apart from the solvent-related factors, the extraction method used plays a role in determining the quantity and type of phytochemicals extracted. This bears implications on the range and magnitude of prospective activities to be observed.
4 EXPERIMENTAL
4.1 Plant materials, reagents, and experimental conditions
Leaves, stems, and barks of 14 plant species (see Table 1) were collected from the botanical garden of the University of Wuerzburg, Germany. Ginger rhizomes were obtained from Kraeuter Mix GmbH, Germany. Fresh plant materials were chopped into small pieces and air-dried at room temperature (26–27°C) for 2 weeks. Dried plant materials were reduced into coarse powders using a laboratory electric blender (Braun, Germany).
Maceration was carried out by soaking plant materials in methanol in a solvent-to-matrix ratio of 10 ml/g. Extraction was done over 24 hr (and over 30 min for 12 control samples) at room temperature under constant stirring by magnetic stirrers. Pressurized MAE was done over 30 min using the same solvent-to-matrix ratio of 10 ml/g, followed by automated stirring, in a microwave reaction system (synthWAVE; MLS GmbH, Germany).[14] Temperature, microwave energy, and pressure were set at 40°C, 150 W, and 10 bar, respectively. The pressure was generated by argon gas. These settings resulted in a temperature–time profile of ambient to 40°C (3 min), 40–45°C (1 min), 45–40°C (7 min), and 40°C (19 min). Automated intermittent microwave irradiation (0–150 W) ensured the maintenance of temperature within the stated limits. Control PMAE experiments at 80°C for 30 min were conducted for 12 control samples in which the temperature rose from ambient to 80°C in 1 min at the microwave energy of 300 W.
After both maceration and PMAE extractions, the crude extracts were filtered (Whatman No. 1) and concentrated under vacuum at 40°C. The resultant semisolid extracts were further freeze-dried for 12 hr, affording dry powders, which were weighed and stored at −15°C.
HPLC analysis was done on an LCMS-2020 (Shimadzu, Japan) equipped with a DGU-20A3R degassing unit, a LC20AB liquid chromatograph, and an SPD-20A UV/Vis detector. Mass spectra were obtained by an LCMS-2020 with a Synergi 4U fusion-RP (150 × 4.6 mm) column as a stationary phase, nebulizing and drying gas (N2) flow rates of 1.5 and 15.0 l/min, respectively, desolvation line temperature of 250°C, and heat block temperature of 400°C. Both systems were controlled by the Shimadzu LabSolutions software. Stock solutions of 20 mg/ml for each extract were prepared using methanol as a diluent. Working solutions at 2 mg/ml were then obtained by further diluting stock solutions using methanol. A method used for routine analyses was applied. The chromatographic conditions entailed a reversed-phase column (RP C18; 4 µm, 150 × 4.6 mm; Agilent Technology) and a mobile phase, solvent A: water + 0.1% formic acid and solvent B: methanol + 0.1% formic acid. Moreover, a gradient elution profile was applied: 5–100% B (0–12 min), 100% B (12–17 min), and 100–5% B (17–18 min). An injection volume of 20 µl was used and UV detection was performed at 254 nm and a tandem ESI–MS operating in positive ionization mode.
4.2 Data analysis
The yields were compared by observing the percentage proportions at which the yield from PMAE differed from those of maceration. This was carried out independently for each sample. For each sample, the UV chromatograms from both methods were superimposed and examined for differences in numbers and intensities of corresponding peaks. 18 MS BPC pairs of corresponding extracts were compared for the presence of the same peaks and the number of additional peaks.
The identity of additional/nonmatching peaks observed in UV chromatograms was cross-checked on the corresponding MS spectra. The peak was regarded as representing an additionally extracted compound if it was not observed in the BPCs of the corresponding extract. The BPCs of each pair of extracts showing additional/nonmatching peaks were comparatively analyzed using box and whisker plots.
ACKNOWLEDGMENTS
The authors would like to acknowledge the technical assistance of Florian Geyer and Christine Heinz. The study was supported by funding from the Deutscher Akademischer Austauschdienst (DAAD) offered to N. E. M. through research grants for Doctoral programmes in Germany, 2018/19 (57381412). Open access funding enabled and organized by Projekt DEAL.
CONFLICTS OF INTERESTS
The authors declare that there are no conflicts of interests.




