Recent progress of biomass based self-healing polymers
Yu Bei and Yufeng Ma contributed equally to this work.
Funding information: The Fundamental Research Funds from Jiangsu Province Biomass and Materials Laboratory, Grant/Award Numbers: JSBEM-S-202001, JSBEM-S-202006; The National Natural Science Foundation of China, Grant/Award Number: 31901256; The Natural Science Foundation of Jiangsu Province, Grant/Award Number: BK20201128
Abstract
Self-healing polymer materials have been developed rapidly over the past 20 years, which can replace thermosetting materials to a certain extent, repair damage without manual intervention, prolong service life, and compress usage cost. The self-healing polymer materials derived from biomass resources can reduce the dependence on traditional non-renewable resources such as petroleum. The structures and properties of biomass based self-healing polymer materials are of great significance for their processing, recycling and self-healing. In this paper, the research progress of bio-based self-healing polymer materials based on dynamic non-covalent bonds and dynamic covalent bonds including hydroxyl ester, Schiff base, disulfide bond, and hydroxyl urethane derived from vegetable oil, lignin, cellulose, vanillin and natural rubber were reviewed, and the dynamic non-covalent bonds and dynamic covalent bonds were also introduced. The applications of various dynamic covalent bonds and dynamic non-covalent bonds in the design and construction of polymer materials were reviewed, and the future development of dynamic covalent polymer materials was prospected.
1 INTRODUCTION
Petroleum-based polymer materials have many excellent properties, which are widely used in industry. The cross-linking network of thermosetting materials limits the mobility at the molecular level and cannot be repeated processing and utilization.1 In the process of production and transportation, the inevitable macroscopic or microscopic damage caused by collision2 will reduce the service life.3 The ideal chemical recovery route is very complex and difficult to industrialize,4-6 which cause environmental pollution when thermosetting materials are discarded and reused.7 The preparation of polymer materials derived from sustainable biomass resources such as cellulose,8-10 lignin,11-13 natural rubber,14-16 and vegetable oil17-19 has been widely reported. Dynamic non-covalent or covalent bonds are introduced simultaneously to endow the material with self-healing properties, and self-healing materials come into being.20-23 It can occur reversible reaction under external stimulation such as heating, even without stimulation,24, 25 which is beneficial to prolong the service life of materials, reduce environmental pressure and dependence on oil resources.
2 DYNAMIC NON-COVALENT BOND
Self-healing polymer materials based on dynamic non-covalent bonds can be efficiently repaired at room temperature, and few or no external energy is needed, which can save resources to a large extent.
2.1 Host-guest interaction
Host–guest interactions are one of noncovalent interactions that enable easy introduction of various functions into macromolecular building blocks allows the formation of complex macromolecular structures with predefined functions, such as self-healing abilities.26
The composite PAcγCD-Dod-HEA was formed by the host-guest interaction between peracetylated γ-cyclodextrin (PAcγCD) and carboxyl-terminated alkyl chain derivative 12-acrylamidododecane acid (Dod) in 2-hydroxyethyl acrylate (HEA) monomer. The introduction of host-guest interaction made the composite PAcγCD-Dod-HEA-CAC based on citric acid modified cellulose (CAC) have self-healing properties. The self-healing efficiency of PAcγCD-Dod-HEA-CAC (1,5) (containing 5 wt% CAC) was 56% at room temperature, and was 84% at 80°C. The tensile test showed that the composite containing CAC had higher healing efficiency. And two kinds of non-covalent bonds produced from cyclodextrin derivatives as host unit and alkyl derivatives as guest unit could be interacted to make self-healing properties.27
In addition to cyclodextrin systems, crown ether systems28-30 and other self-healing materials based on reversible host-guest interactions have been prepared.
2.2 Hydrogen bond
Hydrogen bond, as a common dynamic force, is often used to construct self-healing systems.31-34
The hyperbranched structure composed of phenylpropanoid units and different functional groups in lignin35 limits the synthesis of compatible lignin copolymers, it has excellent adhesion properties after intensive grafting.36 Wang37 designed lignin-graft-poly (n-butyl acrylate-co-1-vinylimidazole) copolymer (lignin-BVs) as an adhesive. Besides strong adhesion and skin adhesion, it also had room temperature self-healing ability. Especially when 19.4 wt% 1- vinylimidazole (VI) was added, lignin-BV19.4 could be self-healing at room temperature. The specific experiment was to cut the sample half with a blade and contact the fresh section. The sample was healed after 1 h, and the self-healing efficiency increased with the increase of healing time. After 4 h of healing, the tensile strength and fracture healing efficiency reached 95% and 94%, respectively.
The hydrogen bond supramolecular hybrid network ENR/CMCS was constructed by introducing carboxymethyl chitosan (CMCS) into epoxy natural rubber (ENR). ENR-10CMCS (ENR containing 10 wt% CMCS) was cut, and the two parts were immediately contacted. After self-healing at room temperature for 12 h, ENR-10CMCS showed better self-healing efficiency than pure ENR. In addition to the auxiliary function of self-adhesion of ENR matrix, dynamic hydrogen bond interaction dominates the self-healing of ENR/CMCS composites.38
Microcapsules containing self-healing solution have been applied to polymer materials such as resin, providing a new idea for the traditional self-healing system.19, 39 When micro cracks occurred, SPI-PLGA-MC (Microencapsulation of poly (d, l-lactide-co-glycolide) (PLGA) containing soy protein isolate (SPI) by adding microfibrillar cellulose (MFC)) could release the healing agent SPI, and bridge it with excessive crosslinking agent, so as to achieve self-healing via strong hydrogen bond formation. The average fracture toughness of self-healing soy protein isolate composite was also significantly higher than that of pure SPI resin. The self-healing SPI composite (containing 15 wt% SPI-PLGA-MCs and 10 wt% MFC) showed a fracture stress of 4 (± 0.3) MPa after 24 h of healing, while the original self-healing composite showed a fracture stress of 15 (± 2) MPa after 0 h, indicating a healing efficiency of 27 (± 4) %.40
The self-healing efficiency of hydrogel is still in control of hydrogen bond as a kind of hydrophilic three-dimensional network polymer. Self-healing hydrogel AG-UPy was designed by Balavigneswaran et al.33 Which was composed of oxidized alginate and urea-pyrimidinone functionalized gelatin (G-UPy), and had two repairing mechanisms of Schiff base formation and UPy dimerization. Comparing AG-Upy with oxidized alginate-gelatin (AG) hydrogel, AG-Upy showed effective self-healing efficiency after 800% strain was applied for about 2 min, while AG could not recover. AG-Upy/PEG-PU/C was prepared by impregnating poly (ethylene glycol)-poly(carbamate) chlorosilane nanohybrid (PEG-PU/C), which also showed rapid self-healing efficiency due to the increase of hydrophobicity. However, with the increase of hydrophobicity, the steric hindrance was increased and the self-healing efficiency was decreased.
2.3 Hydrophobic interaction
Reversible hydrophobic interaction endows hydrogels with self-healing without external stimuli.41, 42 Meng43 used alginate (SA) and silk fibroin (RSF) with stearic methacrylate (C18M) as hydrophobic monomer as surfactant micelles to design a self-healing hydrophobic association (HA) hydrogel. The healing process of SA-RSF-C18M-30 HA hydrogel was observed under polarizing microscope. The broken SA-RSF-C18M-30 HA hydrogel (adding 30 mM/L C18M) healed the cracks into round holes, then the micelles flowed between layers, and the cracks in the scratch was completely healed within 12 h.
2.4 Electrostatic interaction
Electrostatic interaction force is the most effective non-covalent bond film-forming force in the construction of polymer films, which is widely used in the layer-by-layer assembly between polymer ions.44
The appearance of layer-by-layer (LbL) film is not only conducive to improving the strength of composite materials, but also can be used to prepare exogenous self-healing coatings.45 Merindol et al.46 assembled the nanofibrillated cellulose (CNF) with anionic group (carboxyl) and weak cationic polyethylene amine (PVAm) with LbL, and the obtained film was wetted with ultrapure water. The surfaces of the two wet films were overlapped and dried to form a strip repair film for tensile test. It was found that the fracture stress of the repaired membrane (157 ± 33 MPa) was equivalent to that of the original membrane (174 ± 20 MPa). Through the study of image correlation, the deformation of overlapping part was also much smaller than that of other parts of the film, which was indicated that the self-healing behavior of the repaired film occurs. The repair performance observed under wet conditions was accomplished by electrostatic interaction of polyelectrolytes on the surface of multilayer films in water.
3 DYNAMIC COVALENT BOND
In the presence of light, heat and force, the dynamic covalent bond breaking reorganization shows self-healing performance, and the dynamic covalent bond polymer can also show high stability after the elimination of external stimuli. There are many kinds of dynamic covalent bonds. This paper gives examples and introduces them.
3.1 Hydroxy-ester
The dynamic hydroxyl-ester exchange reaction occurs at high temperature, so as to realize the interconnection network rearrangement and endow the material with self-healing and reprocessing properties. The preparation of hydroxyl-ester self-healing materials often requires the addition of Lewis acids such as zinc acetate and zinc acetylacetonate,47, 48 and tertiary amine compounds such as 1,8-diazabicyclo [5.4.0] undec-7-ene (DBU).49, 50
3.1.1 Vegetable oil
The introduction of microwave technology into the dynamic covalent bonding experiment can greatly shorten the reaction time and improve the yield of polymer.51 In Zhang's experiment,52 a novel TO-based UV-curable oligomer (TMG) was synthesized in 25 min by a simple two-step method, in which tung oil (TO) reacted with maleic anhydride (MA) and then further modified with glycidyl methacrylate (GMA). At the same time, a malic acid-based UV curing monomer (MM) was synthesized under environmental conditions, which was used as an active diluent for photopolymerization with TMG to synthesize UV curing materials. In addition, the addition of zinc acetylacetonatehydrate (Zn [aca]2) catalyst can activate the dynamic transesterification (TER) at elevated temperature, which was helpful for the self-healing of UV-curing materials. The scratches of TMG-M1 (containing 9 g TMG, 1 g MM, 0.68 g Zn [aca]2) film were scratched on the surface with a blade, and the recovery rate was 92.5% at 180°C for 1 h. The TMG-M1 strip sample was completely cut and placed at 180°C, and it was basically healed in 10 min, with only a slight healing trace. The tensile strength of the sample after healing could even exceed that of the original sample.
To improve the performance of vegetable oil (VO) derivatives, Li53 prepared a catalyst-free vitreous elastomer TGDDM/DA (TD) based on VO by curing dimer acid (DA) derived from VO and tetrafunctional glycidyl amine epoxy resin (TGDDM). The reaction mechanism and process were shown in Figure 1. Cracks were cut on the surface of TD elastomer by blade, and healed at 200°C for different time periods. The crack width was detected by optical microscope to test the self-healing performance. After healing for 10 min, the crack widths of TD-1/1 and TD-1/1.2 films decreased by 14.0% and 44.1%, respectively. After 30 min, the crack widths of TD-1/1 and TD-1/1.2 films decreased by 29.8% and 67.8%, respectively.
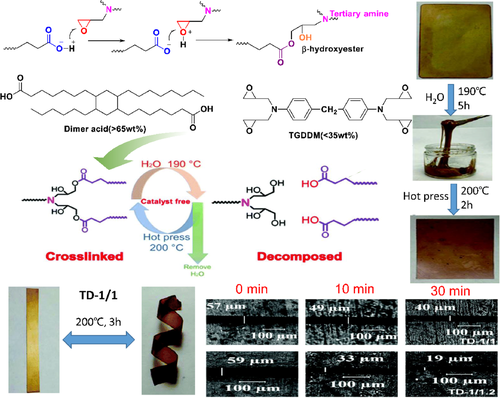
3.1.2 Lignin
Lignin widely exists in nature, and it has become a new trend to introduce it into polymer materials.54-57 So as to solve the problem of incompatibility with resin caused by lignin hyperbranched structure, lignin molecules are modified to improve the solubility of lignin.
Kraft lignin (KL) was oxidized by ozone, the benzene ring with methoxy and hydroxyl groups was opened to form carboxyl groups, and then cured with sebacic acid epoxy resin to prepare hydroxyl-ester vitrimer. The formation of cross-linked network made the lignin-based vitrimers self-healing at high temperature.58
In addition, KL was modified with anhydride to obtain more carboxyl groups, and then cured with poly (ethylene glycol) diglycidyl ether (PEG-epoxy) to prepare coating (lignin-PEG) with high lignin content (>47 wt%). Dynamic transesterification was activated above 140°C, giving lignin-PEG excellent repairable properties. With the assistance of ethylene glycol, the repair efficiency of the coating could reach 50%, and the fissure was repaired from 36 to 18 μm.59
3.1.3 Cellulose
Cellulose is abundant in plants, and is widely used in paper making and other industries. A large number of hydroxyl groups in cellulose can participate in dynamic transesterification.
Zhao60 developed a new vitrimer composite (VC) with polycarbonate as matrix and natural cellulose paper as reinforcement skeleton. The self-healing performance of VC film was studied by examining the change of surface scratch size with time at 160°C. The VC was scratched with a blade and monitored by polarizing microscope. It was found that the width of the crack could heal at least 75% within 10 s because of the exchange reaction between hydroxyl and carbonate at the interface at high temperature, resulting in the rapid formation of reversible covalent bonds.
3.1.4 Natural rubber
Natural rubber is extracted from rubber trees, and the cross-linked network obtained after oxidation or vulcanization is stable. The introduction of hydroxyl-ester dynamic system to develop rubber products can endow them with self-healing properties.
ENR-based vitrimer TCR was prepared by introducing hydroxyl-functionalized CNs (TOCNs).61 Using tuna acid as the cellulose source, a large number of carboxyl groups were introduced by TEMPO-mediated oxidation, which could not only be used as the reinforcing filler of ENR, but also be used as a cross-linking agent by epoxy acid reaction to generate dynamic β-hydroxy ester bonds at the interface, thus giving TCR reusability and reprocessing. The TCR-20 (epoxy/carboxyl molar ratio of 23.2) sample was cut into small pieces and pressed at 180°C for 40 min under 15 MPa pressure. The integrity of TCR-20 was restored, and the recovery rate of tensile strength and fracture strain reached 83% and 90% of the original sample. The samples were cut off and healed at 160°C for 3 h, which could withstand large strain, indicating that TCR has excellent self-healing ability. The healing efficiency based on tensile strength increased with the prolongation of healing time, reaching 80% after 3 h. It was proved that the acceleration of ester exchange at higher temperature would promote the healing process.
3.1.5 Other bio-hydroxy-esters
Polymethyl methacrylate (PMMA) epoxy vitreous composites were prepared by added methyl methacrylate (MMA) and benzoyl peroxide (BPO) into the curing system of epoxy resin and citric acid (CA) monohydrate. When the content of PMMA was 10~25 wt%, PMMA was uniformly dispersed in the matrix and forms a network structure, which improved the transesterification rate of vitrimer, thus improving the self-healing strength and recoverability.62
In the cause of preparing high Tg and repairable bio-based epoxy vitrimer, vanillin was introduced into guaiacol to obtain triphenol compound (main product), was epoxidized to obtain TEP. Epoxy vitrimer was prepared by curing reaction between TEP and 4 - methylcyclohexane-1, 2 - dicarboxylic anhydride (MHHPA).63 Figure 2 shows the total reaction process. Re-arrangement of the cross-linked network endowed the materials with reparability. The film samples were scratched and clamped between the metal plates, and the external force was applied to cause cracks. The crack width of TEP-1 was reduced by more than 70% in 5 min, and reflected its excellent reparability. If TEP-2 was placed in a hot press at 220°C, the crack width was reduced by 90% in 5 min, which was much faster than the result without external stress.
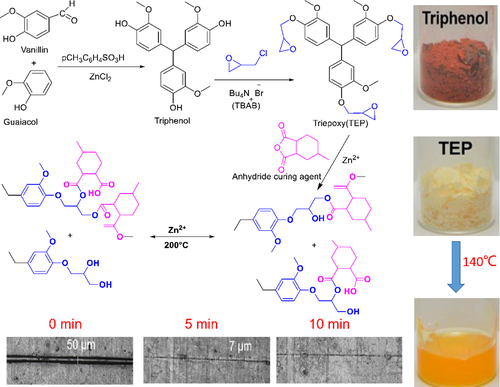
3.2 Schiff base
Dynamic imine bond can be obtained without expensive catalyst in Schiff base reaction.64 Schiff base is generally prepared by aldehyde or ketone and amine,65, 66 and the exchange reaction is adjusted according to the ratio of raw materials.
3.2.1 Vanilla-based polymer
As a kind of natural aldehyde, vanillin is widely distributed in vanilla and eucalyptus leaves, especially in the clip of vanillin. Vanillin is applied to the production of valuable monomers due to its high activity of phenolic hydroxyl and aldehyde groups, and materials with different mechanical properties such as self-healing materials are synthesized.67, 68
Cross-linked polyimine/polyschiff base vitrimer was obtained by imidization of dialdehyde monomer prepared from vanillin (DAV).69 The key part of the process was shown in Figure 3. The thermal reversibility of the imide bond endowed the vitreous with self-healing ability. In order to study the self-healing characteristics, sample I (containing 1.8 mmol diethyltriamine and 2.8 mmol tris [2-aminoethyl] amine) with dog bone shape was cut with scissors. The two overlapping 2 × 3 mm slices were aligned and healed at 150°C. The tensile strength and elongation at break were restored to 74.5% and 118.5% of the original sample, respectively, after 1 h.
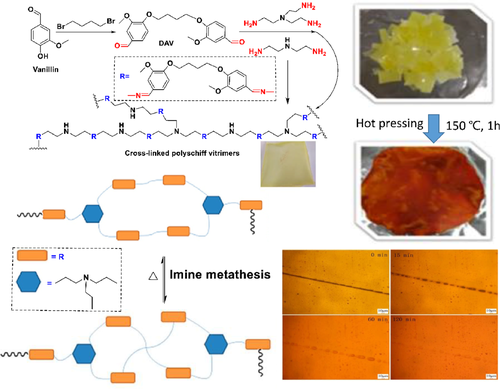
The ester group and epoxy group contained in epoxidized soybean oil (ESO) were subjected to ammonolysis and ring-opening reaction with polyamine compounds to prepare polymer (AESO). Due to its soft linear covalent chain and unstable hydrogen bonds, the practical application of AESO was not high. Therefore, trivanillyl phosphate (TVP) prepared with phosphorus oxychloride and vanillin was introduced to prepare the dynamic polymer (TAESO).70 The hydrogen bond was partially replaced by the dynamic bond formed by aldehydes and amino groups to strengthen the structure and to endow it with high self-healing efficiency. For studying the polymerization performance of TAESO, the dumbbell-shaped TAESO-2.5 (containing 2.5 wt% TVP) sample was cut into two separate parts, connected in a 9 mm2 square overlap at different temperatures. The results showed that the healing efficiency of TAESO-2.5 was proportional to the increase of temperature and time. The healing efficiency of TAESO-2.5 was 93.2% when completely fractured for 12 h and the ambient temperature reached 100°C. The fracture surface could heal even in 1 min under the action of acetic acid, and could support its own weight, and could undergo multiple fracture healing, because the free group produced by the dissociation of the imine bond under the action of acid could be re-formed after the evaporation of acetic acid.
3.2.2 Other bio-based Schiff
In khamrai's experiment,71 bacterial cellulose (BC) was isolated from the strain of Glucanoacetobacter xylinus. Carboxymethylated BC(CMBC) would be achieved after BC's hydroxymethylation. Then it was modified with ethylenediamine and reacted with aldehyde modified pectin formed by Schiff base to obtain self-healing layer by layer (L-B-L) transdermal patch. The sharp blade was used to scratch the surface of the film, and the pH 7.4 buffer solution was used to create a neutral environment for potting the scratch area. After incubation at room temperature for 10 min, the scratch was observed under an optical microscope, and the self-healing efficiency was analyzed by AFM. Wound healing was found to be over 90%. When the buffer solution was sprayed, the scratch surface swelled close to the surface, which accelerated the formation of dynamic imine bonds and led to healing (in Figure 4).
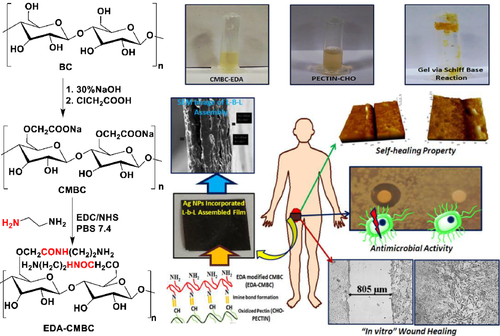
Dynamic covalent fatty polyimine (FPI) was prepared by amination of bio-based dimeric fatty acids in Li′s experiment.72 FPI could heal the surface incision within 24 h at room temperature, and the healing process accelerated with the increase of temperature.
3.2.3 Natural polysaccharide self-healing hydrogel
Natural polysaccharides such as chitosan and hyaluronic acid can be chemically modified to prepare self-healing hydrogels of imine or acylhydrazone systems. Zhang et al.73, 74 synthesized repairable dynamic chitosan-based hydrogels using Schiff base. A dynamic covalent Schiff base was formed between the NH2 group on chitosan (CTS) and the benzaldehyde group at the end of rocker claw bifunctional poly (ethylene glycol) (DF-PEG). The breaking and regeneration of the imine bond endowed the hydrogel with self-healing ability. The procedure of testing about hydrogels' self-healing performance were as follows. First, a radius of 0.5 cm was punched out on the hydrogel disc with a radius of 1 cm, and the holes disappeared after 2 h, which was consistent with the appearance of the initial hydrogel. At the same time, the hydrogel strips were combined into one, and the joint hydrogel strips would not be broken under certain tension.74 Using ethylene glycol chitosan (GCS), which had better solubility, in steading of CTS, and connecting with DF-PEG4000 instantaneously, the obtained hydrogel also had self-healing function.73 Hydrogel GCS-PEG control group experiment was carried out, one of which was stained with Rhodamine B, and the boundaries of two hydrogel discs with different colors were blurred, which proved that they were healing. Compared with ordinary gelatin hydrogel, the pores of self-healing hydrogel disappeared within 15 min after artificial drilling.
In order to eliminate the pain of human wounds in accessories and accelerate the healing of deep wounds, Huang et al.75 developed a nano-composite self-healing hydrogel with water-soluble carboxymethyl chitosan (CMC) and rigid rod-like dialdehyde-modified cellulose nanocrystals (DACNC). The amine in CMC reacted with aldehyde in DACNC to form dynamic Schiff base. Adjusting the molar ratio (MR) of amine to aldehyde could adjust the healing efficiency of hydrogel. One of the two separated CMC-DACNC-48 (MR = 2) hydrogels was stained with Rhodamine B, and the other was stained with methylene blue. The fresh exposed surface could heal within 5 min without external stimulation while two parts approaching enough, and it could withstand sufficient tensile stress at both ends without breaking (Figure 5).
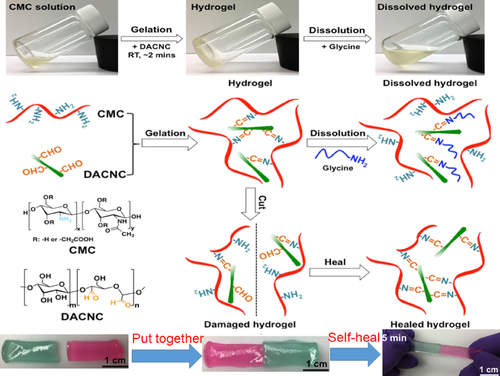
After articular cartilage lesions, due to the lack of blood supply, the self-healing ability is poor. At the same time, with the aim of ameliorating incompatibility and poor integration between biological materials and human tissues, Yu76 introduced the dynamic acylhydrazone bond formed by acylhydrazine and aldehyde to prepare a polysaccharide-based hydrogel for cartilage tissue engineering. Firstly, the carboxyl group of sodium hyaluronate (HA), furfurylamine and the amino group of adipic acid dihydrazide(ADH) were amidated to obtain HA-furan-ADH. At the same time, the furfurylamined HA was reacted with sodium periodate to obtain HA-furan-CHO. Finally, the single cross-linked network (SN) and double cross-linked network (DN) hydrogels were obtained by covalent hydrazone crosslinking of HA-furan-ADH and HA-furan-CHO. And the whole synthesis would be shown as is depicted in Figure 6. SN and DN were cut in half, without external intervention at room temperature, only 3 h to merge. The fracture strength and elongation at break of the hydrogel were tested. It was found that the stress–strain curves of SN and DN after healing were not significantly different from those of the original hydrogel, indicating that the self-healing process can promote the complete recovery of mechanical properties.
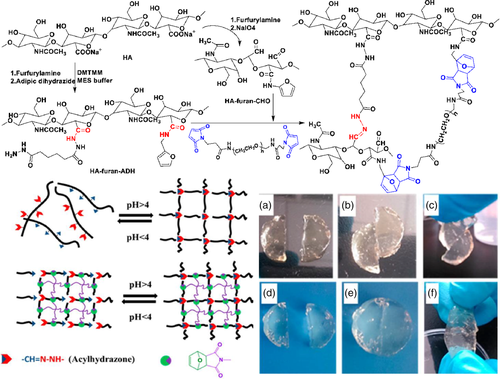
Compared with the ester exchange reaction in the hydroxyl-ester system, the exchange reaction between the imide bonds required a low temperature, which might even occur at room temperature and the reaction rate was fast.
3.3 Disulfide bond
Biopolymers containing dynamic disulfide bonds are different from the original stable molecular networks. Due to the recombination of free radicals, they have self-healing properties.77-81 However, free radicals in the reaction process must be initiated by heating or ultraviolet irradiation, which relies on activators (such as zinc oxide and stearic acid) and accelerators (such as N-Cyclohexyl-2-benzothiazolesulfenamide)82 or even does not need catalysts. For example, copper chloride (CuCl2)-based catalyst was used to activate disulfide or polysulfide bonds in sulfur-crosslinked polybutadiene rubber (BR) at high temperature.83 After stopping heating, the original mechanical properties of BR were restored, and BR had a healing effect. This section focused on two bio-based raw materials, vegetable oil, and natural rubber.
3.3.1 Natural rubber
Natural rubber is soft and easy to break, and the introduction of sulfur element to establish dynamic disulfide bond and polysulfide bond can enhance its mechanical properties and give it healing ability. Three conventional vulcanized (CV) natural rubbers were prepared by Hernández et al.82 according to different sulfur content. The dynamic disulfide bonds could be rearranged at the healing interface after heat treatment, and the mechanical properties could be completely restored without damaging the main chain. At the same time, the longer the thermal healing time of CV2, the more fully the interface contact, the higher the healing efficiency.
In the selection of catalysts, copper methacrylate (MA-Cu) is more suitable for the disulfide bond exchange reaction of vulcanized natural rubber than copper CuCl2. Xiang84 designed related experiments. Compared with inorganic CuCl2, the organic components in MA-Cu had higher compatibility with rubber, which was beneficial to promote the self-healing of vulcanized rubber. The corresponding vulcanized rubber NR-MA-Cu was broken and repaired at 120°C to restore a certain strength. With the extension of recovery time, the tensile strength increased, which could be restored to 78% of the original material strength.
3.3.2 Vegetable oil
Waterborne polyurethane WPUs is considered to be a more environmentally friendly coating. The castor oil contains active hydroxyl, which can be regarded as polyols in the synthesis route of WPUs. Self-healing castor oil based WPU films were prepared by chain extension of 2-aminophenyl disulfide by Lu et al.85 The complete reaction process was shown in Figure 7. The chain extender 2-aminophenyl disulfide gave reversible dynamic polymer networks to heal within 5 min at 60°C.
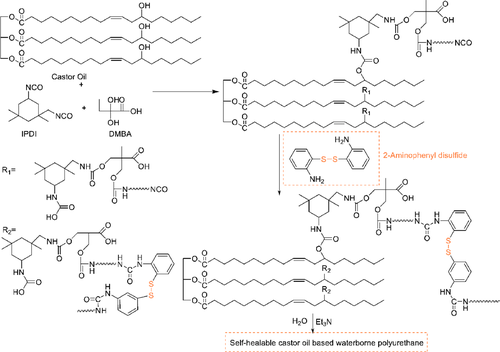
3.3.3 Other bio-based material
Ma et al.86 prepared biobased vitrimer (MDS-EPO) constructed from isosorbide-derived epoxy (IS-EPO) and aromatic diamines (4,4′-disulfanediyldianiline [MDS]) containing disulfide bonds. When MDS-EPO sample had cracks or small damage, heating to 100°C (much higher than the Tg of MDS-EPO) made the dynamic disulfide bond exchange, which could reconnect the fractured surface within 60 min.
Benzoxazine monomer was synthesized from cardanol (CAR) with long alkyl chain and 4- aminophenyl disulfide (4apds), and vitrimer poly (CAR-4apds) containing dynamic disulfide bond was finally prepared.87 Poly (CAR-4apds) films (5 × 5 cm2) were placed directly between two aluminum cubes for tensile test. After fracture, poly (CAR-4apds) films were heated at 120°C for 10 min under 1 bar pressure for healing. It was also found that the thin poly (CAR-4apds) attached to the aluminum surface made the healing process more efficient.
3.4 Hydroxy-urethane
A dynamic covalent bond is also constituted by the exchange reaction between carbamates or the exchange reaction between carbamates and hydroxyls.
The three long chains of tung oil have conjugated triple bonds, which can be used to synthesize tung-oil-based polyphenols (ATOM) by diene with 4-maleimidophenol. At the same time, polyurethane pre-polymer (PTHFTDI) was obtained by using the principle that isocyanate group and phenolic hydroxyl group were crosslinked to form carbamate. Thermosetting polyurethane PTHFTDI-ATOM with self-healing ability was further synthesized using ATOM and prepolymer as raw materials. Studies showed that the broken carbamate bond at high temperature could be recombined at low temperature, thus giving PTHFTDI-ATOM self-healing performance.88
The castor oil-based waterborne polyurethane (WPU) film was developed by 2,2-dihydroxymethylbutyrate (DMBA) and isophorone diisocyanate (IPDI), which showed self-healing due to the crosslinking and recombination of carbamate bonds (Figure 8). The addition of 2,2′-dithiodianiline (DTDA) as a hard segment provided a dynamic disulfide bond for WPU, which improved mechanical properties and self-healing performance. The film with thickness of 0.8 mm was cut into two pieces and placed at 60°C for 6 h. At this time, deep scratches on PU-DTDA0 (without DTDA) were not completely cured, but 500 g objects were lifted without breaking. The films with DTDA such as PU-DTDA20 (functional group ratio, NH2 in DTDA:OH in castor oil = 0.2:0.8) were reprocessed 4 times (140°C, 50 MPa, 1 h) and self-healing at 100°C for 3 h, and the recovery and healing efficiency of tensile strength were close to 100%.89
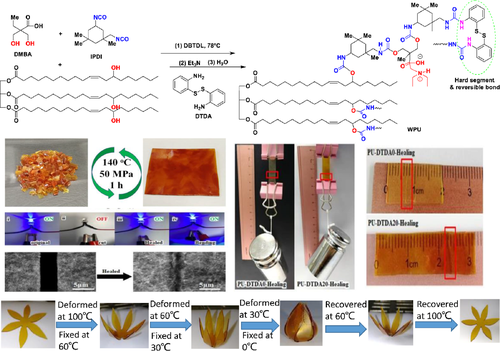
3.5 Dynamic covalent C═C bond
Self-healing hydrogel can be prepared based on dynamic covalent C═C bond formed by Knoevenagel condensation reaction between poly (ethylene glycol) dicyandiacetate and water-soluble poly (vanillin acrylate). The dynamic covalent C═C bond in aqueous solution is thermally reversible. Precursor (PEG2000DCA) was synthesized by esterification of polyethylene glycol (PEG2000) with cyanoacetic acid, then precursor P(DMA-VA) was synthesized using vanillin acrylate (VA) and N,N-dimethylacrylamide(DMA). The phosphate-buffered saline(PBS) solution of two precursors (molar ratio 1:2) was mixed to prepare hydrogel K-gel-PBS. The crucial reaction procedures were shown in Figure 9. One of the K-gel-PBS discs was dyed with methylene blue, the other was not dyed and cut into two halves. Then the semi-discs with different colors were closely bonded along the cutting line, and placed in the original mold at 37°C. It was clearly observed that the hydrogel completely healed and was withstood its own weight without breaking.90

3.6 Borate ester bond
Reversible borate ester bonds can be formed using boric acid and diol in aqueous solution by complexation.91 Hyaluronic acid was obtained by the modification of maltose, and dynamic borate ester bond was generated by the reaction of phenylboronic acid and diol on the maltose. The obtained hydrogel was re-contacted after cutting, and self-healing could be achieved within a few minutes.92
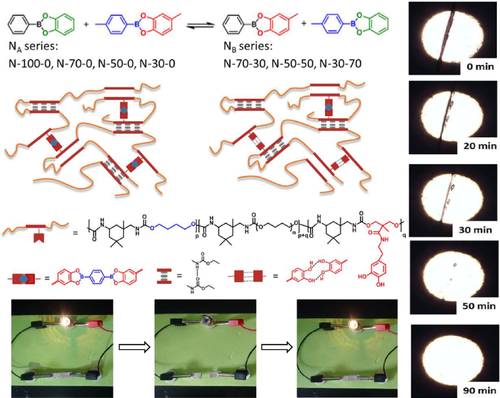
By solution casting method, Yang et al.93 introduced the borate ester crosslinking agent derived from catechol and 1,4 - phenylenediboronic acid into linear polyurethane PU to prepare new elastic vitrimer with different proportions of catechol and different crosslinking densities. The self-healing principle was that catechol-based borates could be hydrolyzed and reformed reversibly in the presence of water. In order to prove its self-healing ability, a small scratch was cut on the surface of N-50-0 (NA type [Figure 10], linear PU-50, with the same ratio of boric acid group in crosslinking agent and catechol group in PU-50) sample with a blade, and the scratch disappeared after 1 h at 25°C by optical microscope monitoring. The dumbbell-shaped N-50-0 sample was cut into two pieces, and the fracture surface was gently contacted without any pressure. Uniaxial tensile tests of original and self-healing samples were performed at different healing time. The healing efficiency increased over time, reaching 94% of the original toughness after 1 h of healing under environmental conditions. By contrast, even at higher temperatures (100°C), the PU elastomer with stable covalent joints could not be repaired.
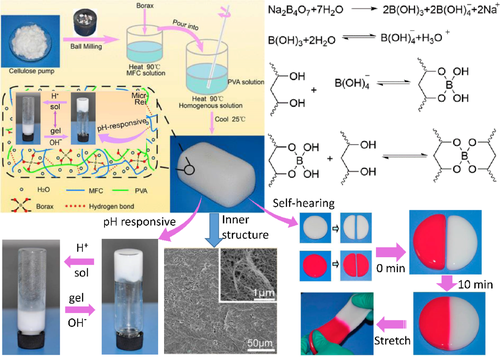
Borate ester-diols (borax and polyvinyl alcohol) can also produce similar structures. The preparation of dynamic self-healing borax hydrogel (PB) using bamboo pulp, polyvinyl alcohol (PVA) and borax as raw materials was very environmentally friendly. In order to study the effect of microfibrillated cellulose (MFC) on the performance of hydrogels, Lu et al.94 introduced it into hydrogel to prepare PB-MFC (Figure 11.) Two new hydrogels PB-MFC-3.0 (containing 3 wt% MFC) with different colors were cut into two pieces. Different colors were in contact with each other. After 10 min of contact, the fracture surface adhered to each other, the hydrogel was actively healed into a single hydrogel without any external stimulation. The hydrogel was firmly stretched by the hand and cracks were generated. In rheological analysis, PB-MFC-3.0 was cut into eight pieces, and the storage modulus G of the broken hydrogel increased from a low value to almost the same as that of the original hydrogel with the extension of time, indicating that the internal recovery was completed.
A simple method for preparing starch-based (starch/polyvinyl alcohol (PVA)/borax, SPB) conductive hydrogel was proposed to promote self-healing of sensitive ion sensors. Due to the double reversible interaction of borate bond and hydrogen bond, the hydrogel exhibits ultra-fast self-healing ability in air and underwater. The stained SPB hydrogel was cut into two pieces, and then the two parts of different colors were in contact with each other in the air. It could be quickly reconnected within 10 s without external stimulation, and could support the total amount of itself. The stress–strain curve of the hydrogel after healing was almost the same as that of the original hydrogel, and the self-healing efficiency could reach 94.3%. In addition, two hydrogels of different colors were combined underwater and fully contacted. After 120 s, they could withstand a certain degree of stretching under water.95
3.7 Metal coordination bond
The dynamic and reversible metal coordination bond endows the material with stimulus,96 while repeated fracture and healing at the molecular level can effectively dissipate energy. Therefore, the introduction of metal-coordination bond into the material improves the self-healing performance as well as mechanical strength of the composites.97
A self-healing ionic gel was designed by constructing coordinated multiple coordination bonds between tannic acid-coated cellulose nanocrystals (TA@CNCs), poly (acrylic acid) chains and metal ions. The dynamic coordinated interaction caused by multiple coordination bonds gives the self-healing ability of the gel without external stimulation. The specific operation is as follows: The rectangular samples were cut into two halves. They were immediately contacted and sealed without external force. The early healing efficiency increased with the prolongation of healing time. The healing efficiency rapidly increased to about 88% in 20 min, the healing efficiency reached 92% after 30 min, which was in a balanced state. The results showed that the increase of TA@CNC content could effectively improve the self-healing efficiency of ionic gels.98
In order to solve the problem of the lack self-healing ability of traditional hydrogels, a polyacrylic acid/nanochitin composite hydrogel with double cross-linked network was synthesized.99 The hydrogel was attached immediately after being cut, and the tensile strength increased with the extension of the healing time. When the healing reached 2 h, the self-healing efficiency could reach more than 97%. Optical microscope and scanning electron microscope were used to observe the incision morphology of PAA/NCT hydrogel. It was found that the incision gradually decreased with the extension of the self-healing time.
3.8 Diels-Alder (D-A) bond
The formation of reversible dynamic cross linkages by the Diels-Alder (DA) reaction between furan and maleimide groups is one of the most extensively investigated in the study of self-healing polymers.100
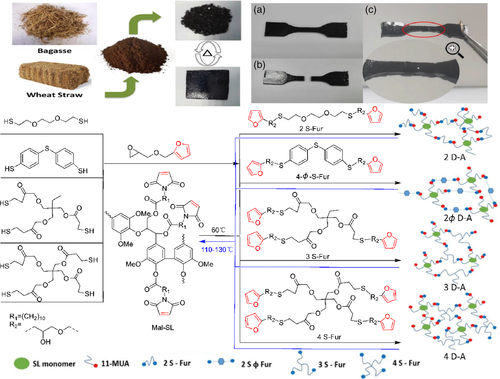
In the absence of solvent and catalyst, soda lignin (SL) derivative material containing maleimide (Mal) group and four multi-functional furan connectors (S-Fur) was obtained by D-A polymerization of furan/maleimide.100 After only 1 h at 60°C, the self-healing process of the material was obvious. No further healing was observed after 24 h at the same temperature, but further healing could be achieved by warming. After 20 min at 110°C, 2S-Fur-Mal-SL almost completely recovered. With the purpose of macroscopically confirmation the healing ability of 2 D-A and 4 D-A, the dumbbell-shaped samples were cut into two pieces and placed in contact. The dumbbell-shaped samples were healed at 120°C for 20 min and cured at 60°C be recombined, and the possibility of adhesion healing caused by non-covalent bonds was excluded (Figure 12).
4 CONCLUSION
A variety of biomass materials and their derivatives, such as vegetable oil, lignin, cellulose, vanillin and natural rubber have been widely used in self-healing polymer materials. By introducing dynamic covalent or non-covalent bonds in the biomass based cross-linked polymer network, the self-healing ability is obtained and can prolong the service life of the material and reduce waste. Most of the available biomass raw materials have complex structures, so preparing biomass compounds with single structure and easy control of reaction through effective methods has become a research hotspot. However, the use of edible biomass resources may occupy food sources. Dynamic covalent bonds such as disulfide bonds and imine bonds have lower energy than covalent bonds such as carbon–carbon bonds and carbon-nitrogen bonds, which are more prone to decomposition or reversible reactions, but also sacrifice the thermal stability of the material. The reprocessing of most bio-based self-healing polymer materials requires harsh conditions (such as high temperature and high pressure), which is difficult to implement in industry. It is believed that with the expansion and deepening of research in this field, bio-based self-healing polymer materials can realize industrial production, which will improve human life and living environment and promote sustainable development.
ACKNOWLEDGEMENTS
This work was supported by the Fundamental Research Funds from Jiangsu Province Biomass and Materials Laboratory (JSBEM-S-202001, JSBEM-S-202006), the Natural Science Foundation of Jiangsu Province (BK20201128) and the National Natural Science Foundation of China (No. 31901256).
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Biographies

Yu Bei is pursuing a master's degree at the College of Chemical Engineering, Nanjing Forestry University. My research focuses on bio-based polymeric materials, especially modified lignin-based flame retardant as well as plant oil-based plasticizer, which catches my interest.

Yufeng Ma earned his B.Sc. in Chemical Engineering and technology in 2006 from Henan University of technology. He earned his M.Sc. in Chemical technology in 2009 from Fuzhou University and Ph.D. in Chemical Processing Engineering of Forest Products in 2013 from the Chinese Academy of Forestry, Beijing, China. Dr. Yufeng Ma is currently an associate professor in Nanjing Forestry University. Dr. Yufeng Ma's current research focuses primarily on novel sustainable bio-based composites with agricultural and forestry resources.

Fei Song is the postdoc at the College of Chemical Engineering, Nanjing Forestry University. Ph.D. from the Institute of Chemical Industry of Forest Products, Chinese Academy of Forestry. B.AgrSc College of Forestry, Jiangxi Agricultural University. B.Sc. Faculty of Science, Jiangxi Agricultural University.

Zhimin Kou pursuing a master's degree in the college of Materials Science and Engineering, Nanjing Forestry University. My research focus is on bio-based self-healing polymer materials, especially modified self-healing polymer based on soybean oil. Now I am further studying in this field

Lihong Hu is a professor in Institute of Chemical Industry of Forest Products, Chinese Academy of Forestry, Nanjing, China. She obtained Ph.D. and M.Sc. from the Chinese Academy of Forestry, Beijing, China. B.Sc. from Henan University, Kaifeng, China.

Caiying Bo is an associate professor in Institute of Chemical Industry of Forest Products, Chinese Academy of Forestry, Nanjing, China. She obtained Ph.D. and M.Sc. from the Chinese Academy of Forestry, Beijing, China. Her research focuses particularly on bio-based polymer materials.

Puyou Jia started his academic career by graduating from Zhengzhou University with a Bachelor of Applied Chemistry in 2009 and the obtained his Master of Physical chemistry from Qinghai University for Nationalities in 2013. He then completed his Doctor of Chemical Processing Engineering of Forest Products in Beijing Forestry University. Dr. Puyou Jia is currently an associate professor in the Institute of Chemical Industry of Forest Products, Chinese Academy of Forestry, Nanjing City.

Yonghong Zhou started his academic career by graduating from Henan University with a Bachelor of Chemistry in 1988 and the obtained his Master and Doctor of Chemical Processing Engineering of Forest Products from Chinese Academy of Forestry in 1991 and 2002. Dr. Yonghong Zhou is currently a professor and director of the Institute of Chemical Industry of Forest Products, Chinese Academy of Forestry, Nanjing City.




