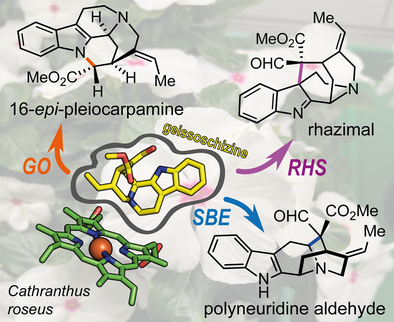
Oxidative Rearrangements of the Alkaloid Intermediate Geissoschizine
Graphical Abstract
19E-geissoschizine is a key precursor in the biosynthesis of monoterpene indole alkaloids such as strychnine, ibogaine, and vinblastine. Using enzymatic assays and gene silencing, three CYPs were identified from C. roseus that oxidize this compound to four alkaloid scaffolds: strychnos, sarpagan, akuammiline, and mavacurane. Mutational analysis reveals how active-site changes fine-tune product specificity, highlighting nature's strategy for metabolic diversity.
Abstract
Plants can generate structural diversity by enzymatic rearrangement of a central intermediate. 19E-geissoschizine is one such chemically versatile intermediate that plays a central role in the biosynthesis of monoterpene indole alkaloids such as strychnine, ibogaine, and vinblastine. Here we report how 19E-geissoschizine undergoes oxidative transformations to generate four distinct alkaloid scaffolds through the action of three biosynthetic enzymes. Using in vitro enzymatic assays and gene silencing, we demonstrate how these three cytochrome P450 enzymes in the medicinal plant Catharanthus roseus transform 19E-geissoschizine into strychnos, sarpagan, akuammiline-type, and mavacurane-type alkaloids. We use mutational analysis to show how minimal changes to the active site of these similar enzymes modulate product specificity. This work highlights how substrate reactivity and enzyme mutations work synergistically to generate chemical diversity.
The monoterpene indole alkaloids are a large family of ca. 3000 structurally diverse alkaloids originating from a single substrate, strictosidine[1] These natural products are of immense pharmacological and synthetic interest. For example, vinblastine and vincristine (anticancer agents), ajmaline (antiarrhythmic), reserpine (neurological activity), and strychnine (poison) are used as medicines, biochemical tools, or inspirations for chemical synthesis.[2, 3] Biosynthetically, strictosidine is deglucosylated by strictosidine glucosidase (SGD) and then subsequently reduced to form 19E-geissoschizine (geissoschizine) by the medium-chain alcohol dehydrogenase geissoschizine synthase (GS).[4-6] Geissoschizine, a corynanthe-type alkaloid is densely functionalized, harboring an alpha-beta unsaturated carbonyl, enol, indole, and basic nitrogen, allowing a wide range of downstream reactions.[7] It serves as the central precursor for strychnos, sarpagan, akuammiline, and mavacurane-type monoterpene indole alkaloids, which exhibit a range of potent bioactivities, and have also been exploited as challenging synthetic targets (Figure 1).[7, 8] Here, we show how three cytochrome P450 enzyme homologs oxidize geissoschizine to form these four distinct alkaloid scaffolds, and how the activity of these enzymes can be interconverted through mutations in the active site. The results demonstrate how nature capitalizes on the inherent reactivity of a highly versatile starting substrate to generate a variety of natural product scaffolds. Given the pharmacological importance of MIAs and the synthetic challenges posed by these intricate scaffolds, this work provides key insights into how nature efficiently constructs these molecules—knowledge that could guide bioengineering efforts and inspire novel synthetic approaches.
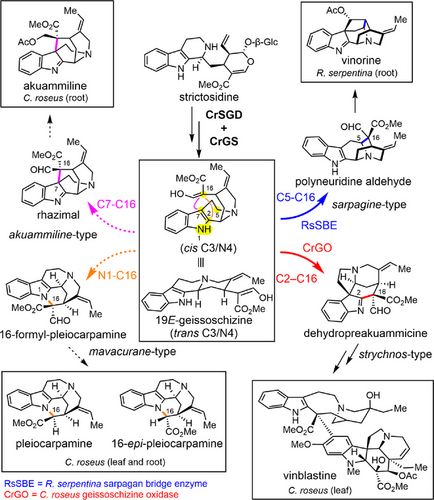
Previously reported enzymes that oxidize geissoschizine belong to the cytochrome P450 CYP71 subfamily.[4, 9-12] The C16-formyl ester moiety of geissoschizine and the C16R stereocenter of geissoschizine play a crucial role in downstream scaffold biosynthesis.[4, 11, 13] The strychnos-type scaffold dehydropreakuammicine is formed by oxidative coupling of C16 and C2 by the Catharanthus roseus CYP71 enzyme CrGO (geissoschizine oxidase, also identified in Strychnos nux vomica),[4, 11] while SBE (sarpagan bridge enzyme), isolated from the related plant Rauvolfia serpentina, catalyzes formation of the C16 and C5 bond to form the sarpagan scaffold, polyneuridine aldehyde[9] (Figure 1). RS (rhazimal synthase), which catalyzes formation of the C16 and C7 bond to form the akuammiline-type alkaloid rhazimal, has been identified in Alstonia scholaris.[11] In principle, the C16 carbon of geissoschizine can also react with the indole nitrogen to form the mavacurane-type alkaloids pleiocarpamine,[7] though an enzyme that catalyzes this transformation has not been identified (Figure 1). The medicinal plant C. roseus is only known for production of alkaloids derived from the GO product, dehydropreakuammicine (strychnos-type).[4] However, we noted that the transcriptome of C. roseus encodes numerous GO homologs with unknown function.
We curated and built a phylogeny consisting of these 16 unknown C. roseus CYP71 genes along with similar P450s of known function (Figure S1). The CYP71 enzymes of unknown function were expressed in Saccharomyces cerevisiae (yeast), and isolated microsomes containing the heterologous P450 were screened for activity with geissoschizine. Candidate CRO_T014436 yielded a major product (m/z 351.1703), along with a minor product (m/z 323.1754) and trace amounts of akuammicine (m/z 323.1754), the known deformylation product of dehydropreakuammicine[4] (strychnos-type) (Figure 2a). To identify the major and minor products, we scaled up the in vitro reaction for product isolation. The minor product was shown by NMR analysis to be the akuammiline-type alkaloid strictamine, which is the deformylation product of rhazimal (see Supporting NMR Data). The major product converted to strictamine over the course of purification, but partial NMR analysis of this unstable product was consistent with a structural assignment of rhazimal (see Supporting NMR Data) (Figure 2a). Therefore, we named this enzyme rhazimal synthase (CrRS). Rhazimal, strictamine, or alkaloids derived from these products have not been reported from C. roseus. However, when we carefully profiled the roots and leaves of C. roseus in search of akuammiline-type alkaloids, we found the rhazimal derived compound, akuammiline, in the roots
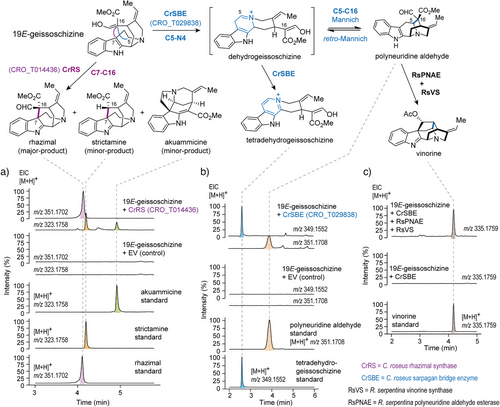
(Figure S2). This suggests that rhazimal in C. roseus is metabolized into akuammiline. In the absence of downstream enzymes, deformylation of rhazimal to strictamine occurs. The in planta function of CrRS could not be definitively established by virus-induced gene silencing (VIGS), since CrRS expressed in the roots and silencing is only possible in C. roseus leaf and stem tissue.
Assay of CRO_T029838 with geissoschizine led to production of an oxidized product that coeluted with a synthetic tetradehydrogeissoschizine (m/z 349.1547) standard. We could also detect small amounts of polyneuridine aldehyde (m/z 351.1703), which also coeluted with the respective standard (Figure 2b). We named this enzyme sarpagan bridge enzyme (CrSBE). Notably, polyneuridine aldehyde appeared to be the initially formed product, but was converted to tetradehydrogeissoschizine over time (Figure S3). Polyneuridine aldehyde synthesis most likely takes place via an initial oxidation of the C5─N4 bond to form an iminium species. Formation of polyneuridine aldehyde involves bond formation between C16 and C5 via a Mannich reaction. We hypothesized that this C5─C16 bond forming reaction can take place in reverse, reverting to the initial oxidation iminium ion product by a retro-Mannich reaction. Therefore, formation of polyneuridine aldehyde would be a reversible process. Formation of tetradehydrogeissoschizine is formed by a second oxidation of the initial iminium intermediate formed by SBE. This process, in contrast, is likely to be irreversible, due to the formation of the highly stable aromatic β-carboline moiety (Figure 2b). Therefore, the initially formed polyneuridine aldehyde product would gradually convert to tetradehydrogeissoschizine over time. To explore how the polyneuridine aldehyde product could be funneled into downstream metabolic processes, we assayed CrSBE in combination with previously characterized enzymes known to convert polyneuridine aldehyde to the stable compound vinorine by the enzymes polyneuridine aldehyde esterase (PNAE)[14] and vinorine synthase (VS)[15, 16] from R. serpentina. The clean formation of vinorine (m/z 335.1752) (Figure 2c) demonstrates how the Mannich reaction is favored when downstream enzymes are present to consume the initially formed polyneuridine aldehyde. Notably, C. roseus produces no detectable products derived from polyneuridine aldehyde, dehydrogeissoschizine, or tetradehydrogeissoschizine. We speculated that CrSBE may oxidize the corynanthe alkaloids ajmalicine/tetrahydroalstonine, substrates that are structurally similar to geissoschizine, in planta. However, when CrSBE was silenced in C. roseus leaves by VIGS, there was no significant reduction in the levels of the oxidized products (serpentine/alstonine) (Figure S4). The physiological function of CrSBE in C. roseus therefore remains unknown.
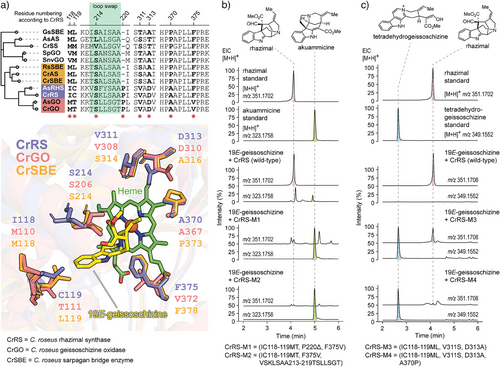
None of the remaining 14 CYP candidates showed activity with geissoschizine. Therefore, with three functional C. roseus CYPs in hand (CrGO, CrRS, CrSBE, and 51%–75% amino acid identity, Figures S5, S6), we set out to understand the molecular basis of this product selectivity. In CrGO and CrRS, “compound I” —an oxoiron(IV) porphyrin cation radical intermediate— would abstract a hydrogen atom from the N─H bond of the indole moiety, generating a C7 radical intermediate (Figure 3, path a). This radical could undergo oxygen rebound with the hydroxoiron(IV) intermediate (“compound II”), leading to C7-hydroxylation of geissoschizine (Figure 3, path b). Subsequent dehydration of the hydroxy group would yield a C7 carbocation intermediate. Alternatively, C7 radical could be directly oxidized to the C7 carbocation via electron transfer with “compound II” (Figure 3, path c). Rhazimal formation likely proceeds via regioselective nucleophilic addition of the C16 enol group to C7, while C2 nucleophilic addition followed by a 1,2-migration leads to dehydropreakuammicine formation. In contrast, CrSBE likely abstracts a hydrogen atom from C5 of geissoschizine (Figure 3, path d), forming a C5 radical intermediate, which would then undergo either oxygen rebound or electron transfer to yield a C5 iminium ion. A subsequent Mannich reaction produces polyneuridine aldehyde, while a second oxidation leads to tetradehydrogeissoschizine. We hypothesize that variations in the active site pockets of these P450s enzymes influence the substrate positioning relative to the catalytic center, thereby controlling regioselective oxidation and subsequent nucleophilic attack. Structural models of CrGO, CrRS, and CrSBE were constructed and compared to identify amino acids that could be responsible for the oxidation and cyclization specificity, which were then targeted for mutational analysis (Figure 4a). Homology models revealed that CrRS and CrGO possess three distinct residues within their active sites (IC118-119MT, F375 V). Although serine at position 214 is conserved in both CrGO and CrRS, its orientation differs significantly. We hypothesize that this serine may form a hydrogen bond with the C16 enol moiety, influencing the C7-C16 and C2-C16 distances and thereby determining the regioselective nucleophilic addition. Reciprocal mutations between residues 213 and 219 in CrRS and CrGO (VSKLSAA213-219TSLLSGT), or the deletion of P220 as observed in CrSBE, may alter the orientation of serine. Using this approach, the native activity of CrRS (production of akuammiline-type alkaloids rhazimal/strictamine) could be converted to GO activity (production of the strychnos-type alkaloids dehydropreakuammicine/akuammicine) as demonstrated by the activity of mutants CrRS-M1 (4 mutations) and CrRS-M2 (10 mutations) (Figure 4b). The native activity of CrRS could also be converted to SBE activity (production of sarpagan-type alkaloid polyneuridine aldehyde along with tetradehydrogeissoschizine) with CrRS M3 (4 mutations) and CrRS-M4 (5 mutations) (Figure 4c). CrGO could be partially switched to CrRS (CrGO-M1) and to CrSBE (CrGO-M2) (Figure S7), while CrSBE could be partially switched to CrRS (CrSBE-M1 and M2) (Figure S7). Conversion of CrSBE to CrGO was not possible. This mutational analysis suggests that the active site of these enzymes can be reshaped with relatively few mutations, which in turns allows the flexible geissoschizine substrate to bind in alternative positions. Subsequent oxidation and cyclization would proceed with altered regioselectivity to generate the three different scaffolds.
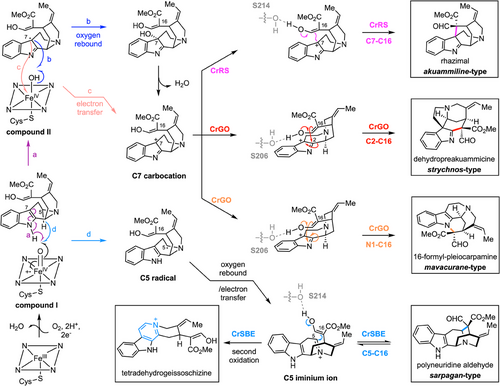
The last missing predicted enzyme would catalyze cyclization via N1─C16 bond formation. This would result in formation of 16-formyl-pleiocarpamine, which, like the RS product rhazimal and the GO product dehydropreakuammicine, is expected to undergo spontaneous deformylation, in this case producing pleiocarpamine and 16-epi-pleiocarpamine[17] (Figure 5a). However, none of the 16 CYP candidates or mutants appeared to produce 16-formyl-pleiocarpamine, pleiocarpamine, or 16-epi-pleiocarpamine. Upon careful inspection, we could detect the presence of pleiocarpamine and 16-epi-pleiocarpamine in C. roseus leaves and root, which strongly suggested that C. roseus should harbor an enzyme with this cyclization specificity (Figures 5b, S8). To our surprise, when we closely examined the product profile of CrGO in vitro reaction assays, trace but detectable amounts of 16-epi-pleiocarpamine along with the major, previously reported product akuammicine[4] were observed (Figure 5c). This minor product shared identical MS2 and retention time to an authentic standard of 16-epi-pleiocarpamine, which suggested deformylation from an initially formed 16-formyl-pleiocarpamine product. The same results were observed when CrGO was expressed in leaves of Nicotiana benthamiana, and disks of these leaf tissues were incubated with geissoschizine (Figure 5c).
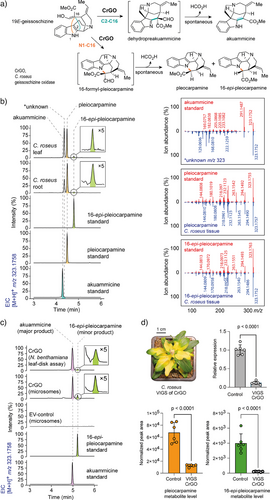
We initially suspected that these low levels of 16-epi-pleocarpamine were simply an artifact of the in vitro enzymatic reaction. However, when we silenced CrGO in C. roseus using VIGS, a significant reduction in the levels of pleiocarpamine and 16-epi-pleocarpamine in C. roseus leaf was observed (Figures 5d, S9). Therefore, the minor product of this in vitro enzymatic reaction may play a physiologically significant role. We propose that 16-formyl-pleiocarpamine forms via nucleophilic attack of the C16 enol group on N1 of the C7 carbocation intermediate (Figure 3, path c). In CrGO, we hypothesize that the serine 206 residue forms a hydrogen bond with the C16 enol moiety, orienting it toward the C2/N1 positions instead of the C7 position. This spatial arrangement favors nucleophilic addition at C2/N1, leading to the formation of both akuammicine and 16-epi-pleiocarpamine. Although 16-epi-pleiocarpamine has been reported as the more thermodynamically stable epimer,[13] the observed differences in product distribution between our in vitro assays (Figure 4c) and in planta systems (Figure 4b) warrant further mechanistic investigation. We speculate that in vitro, deformylation proceeds spontaneously through a thermodynamically controlled pathway, favoring 16-epi-pleiocarpamine formation. In contrast, the plant may employ an enzymatic deformylation that would exert stereochemical control during protonation, selectively yielding the kinetically favored pleiocarpamine. Recently, an ortholog of CrRS in A. scholaris was also shown to generate low amounts of 16-epi-pleiocarpamine though the physiological relevance was not validated by in planta studies.[18]
Here we report the discovery of two cytochrome P450 enzymes, CrRS (rhazimal synthase) and CrSBE (sarpagan bridge enzyme), from C. roseus. Although enzymes with these activities had been identified from other plant species, C. roseus was not reported to have alkaloids derived from the products of these enzymes. Subsequent analysis revealed the presence of a CrRS-derived alkaloid, but the function of CrSBE remains unknown. The sequences of these three enzymes could be compared to demonstrate which residues are responsible for the regioselectivity of the oxidation and cyclization of geissoschizine. Additionally, we show that CrGO (geissoschizine oxidase), in addition to catalyzing C16─C2 bond formation, also catalyzes the C16─N1 bond formation required for the formation of the mavacurane-type alkaloids pleiocarpamine and 16-epi-pleiocarpamine (Figure 1). Mavacurane-type alkaloids have received limited attention[19, 20] but are known to be converted into a range of complex bisindole alkaloids.[7, 21] Silencing of GO in C. roseus strongly suggests that, although pleiocarpamine and 16-epi-pleiocarpamine are only formed as minor products by GO, this enzyme may contribute to mavacurane biosynthesis in the C. roseus plant. This raises the intriguing possibility that the production of minor side products in enzyme reactions can play a significant role in shaping the evolution of metabolic diversity.
Supporting Information
Supplementary Figures S1–S9 and Tables S1–S4, experimental procedures, and compound characterization data is provided. CrRS (PV446799) and CrSBE (PV446798) are deposited.
Acknowledgements
Polyneuridine aldehyde was a generous gift from Dr. Laurent Evanno (Univ. Paris-Sud, CNRS, Université Paris-Saclay, France), 16-epi-pleiocarpamine was a generous gift from Dr. Guillaume Vincent (Univ. Paris-Sud, CNRS, Université Paris-Saclay, France), and pleiocarpamine was a generous gift from Prof. Dr. Hartmut Laatsch (Georg-August-Universität Göttingen, Germany). The authors would like to thank Dr. Yoko Nakamura (MPICE) for NMR measurements. The authors would like to thank Sarah Heinicke (MPICE) for assistance with mass spectrometry. The authors also thank the members of the MPICE Research Greenhouse for their care and provision of Nicotiana benthamiana and Catharanthus roseus plants. The authors acknowledge funding from the Max Planck Society and the Leibniz Prize (505457618).
Open access funding enabled and organized by Projekt DEAL.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.