Light-Driven Site-Selective Glycosylation of Native Carbohydrates
Graphical Abstract
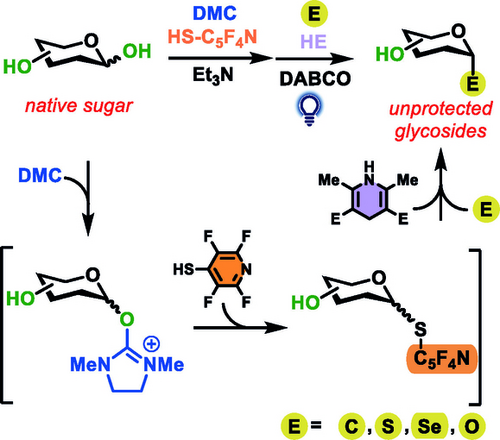
Davis, Koh, and co-workers disclosed a method for transforming native sugars through homolytic cleavage of an isolatable S-glycoside, in notable >95 : 5 α : β stereoselectivity. The sugar is activated by 2-chloro-1,3-dimethylimidazolinium chloride (DMC) for the installation of an S-moiety that undergoes desulfurative C−C coupling using a combination of Hantzsch ester (HE) as reductant and 1,4-diazabicyclo[2.2.2]octane (DABCO) under LED irradiation.
Abstract
Carbohydrates constitute the largest source of biomass on Earth, but their synthetic modification is challenging due to their high content in oxygen functionalities. The site- and stereoselective modification of native sugars is a definite goal of glycochemistry research. Recent efforts to bypass the need for protecting groups, leveraging selective activation through photochemical mechanisms for site-selective C−C bond formation from native sugars, are likely to largely impact all glycochemistry-related areas. Davis, Koh, and co-workers have recently presented their use of photocatalysis to develop a “cap and glycosylate” approach for the site- and stereoselective C-glycosylation of native sugars. A modernized direct radical functionalization of in situ formed thioglycoside using photocatalysis was used in the synthetic manipulation of unprotected carbohydrates. This allowed reaching complex saccharides, and post-translational modification of proteins.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.