Zinc-Ferricyanide Flow Batteries Operating Stably under −10 °C
Liping Zhi
Division of Energy Storage, Dalian National Laboratory for Clean Energy Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 457 Zhongshan Road, Dalian, 116023 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorChenyi Liao
Laboratory of Molecular Modeling and Design, State Key Laboratory of Molecular Reaction Dynamics Dalian Institute of Chemical Physics, Chinese Academy of Science, 457 Zhongshan Road, Dalian, 116023 China
Search for more papers by this authorPengcheng Xu
Division of Energy Storage, Dalian National Laboratory for Clean Energy Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 457 Zhongshan Road, Dalian, 116023 China
Key Laboratory of Long-Duration and Large-Scale Energy Storage, Chinese Academy of Sciences, Dalian, 116023 China
Search for more papers by this authorProf. Guohui Li
Laboratory of Molecular Modeling and Design, State Key Laboratory of Molecular Reaction Dynamics Dalian Institute of Chemical Physics, Chinese Academy of Science, 457 Zhongshan Road, Dalian, 116023 China
Search for more papers by this authorCorresponding Author
Prof. Zhizhang Yuan
Division of Energy Storage, Dalian National Laboratory for Clean Energy Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 457 Zhongshan Road, Dalian, 116023 China
Key Laboratory of Long-Duration and Large-Scale Energy Storage, Chinese Academy of Sciences, Dalian, 116023 China
Search for more papers by this authorCorresponding Author
Prof. Xianfeng Li
Division of Energy Storage, Dalian National Laboratory for Clean Energy Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 457 Zhongshan Road, Dalian, 116023 China
Search for more papers by this authorLiping Zhi
Division of Energy Storage, Dalian National Laboratory for Clean Energy Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 457 Zhongshan Road, Dalian, 116023 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorChenyi Liao
Laboratory of Molecular Modeling and Design, State Key Laboratory of Molecular Reaction Dynamics Dalian Institute of Chemical Physics, Chinese Academy of Science, 457 Zhongshan Road, Dalian, 116023 China
Search for more papers by this authorPengcheng Xu
Division of Energy Storage, Dalian National Laboratory for Clean Energy Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 457 Zhongshan Road, Dalian, 116023 China
Key Laboratory of Long-Duration and Large-Scale Energy Storage, Chinese Academy of Sciences, Dalian, 116023 China
Search for more papers by this authorProf. Guohui Li
Laboratory of Molecular Modeling and Design, State Key Laboratory of Molecular Reaction Dynamics Dalian Institute of Chemical Physics, Chinese Academy of Science, 457 Zhongshan Road, Dalian, 116023 China
Search for more papers by this authorCorresponding Author
Prof. Zhizhang Yuan
Division of Energy Storage, Dalian National Laboratory for Clean Energy Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 457 Zhongshan Road, Dalian, 116023 China
Key Laboratory of Long-Duration and Large-Scale Energy Storage, Chinese Academy of Sciences, Dalian, 116023 China
Search for more papers by this authorCorresponding Author
Prof. Xianfeng Li
Division of Energy Storage, Dalian National Laboratory for Clean Energy Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 457 Zhongshan Road, Dalian, 116023 China
Search for more papers by this authorGraphical Abstract
Abstract
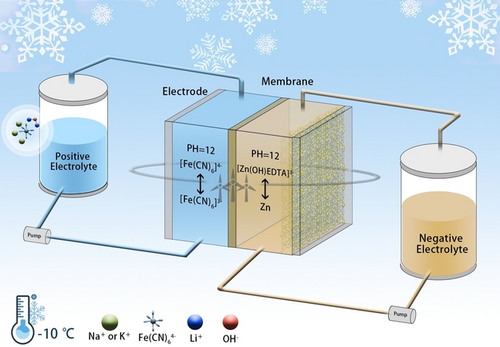
Alkaline ferri/ferro-cyanide-based flow batteries are well suited for energy storage because of their features of high electrochemical activity, good kinetics and low material cost. However, they suffer from low energy density and poor temperature adaptability. The ferri/ferro-cyanide catholyte exhibits low solubility (~0.4 M at 25 °C) in NaOH- or KOH-based supporting electrolyte and can easily form precipitates below room temperature. Here we report a lithium-based supporting electrolyte that significantly enhances the solubility of ferrocyanide. The use of LiOH intensifies the ion-dipole interaction between water molecules and solutes and cripples polarization among ferrocyanide ions. Thus, we have achieved a ferrocyanide-based catholyte of 1.7 M at 25 °C and of 0.8 M at −10 °C. A zinc-ferricyanide flow battery based on the lithium-based supporting electrolyte demonstrates a steady charge energy of ~72 Wh L−1catholyte at 25 °C, and maintains stable for ~4200 cycles (~4200 hours). Furthermore, it remains stable for ~800 cycles (~800 hours) at −10 °C.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202412559-sup-0001-misc_information.pdf1.8 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aH. Brendel, Nature 2022, 608, 667–667;
- 1bH. Thompson, Nature 2022, 603, 364–364.
- 2S. Chu, Y. Cui, N. Liu, Nat. Mater. 2016, 16, 16–22.
- 3
- 3aY. Ding, C. Zhang, L. Zhang, Y. Zhou, G. Yu, Chem 2019, 5, 1964–1987;
- 3bB. Li, Z. Nie, M. Vijayakumar, G. Li, J. Liu, V. Sprenkle, W. Wang, Nat. Commun. 2015, 6, 6303.
- 4
- 4aJ. Cao, M. Tao, H. Chen, J. Xu, Z. Chen, J. Power Sources 2018, 386, 40–46;
- 4bM. Shin, S. Oh, H. Jeong, C. Noh, Y. Chung, J. W. Han, Y. Kwon, Int. J. Energy Res. 2022, 46, 8175–8185;
- 4cL. Zhi, T. Li, X. Liu, Z. Yuan, X. Li, Nano Energy 2022, 102, 107697;
- 4dZ. Liu, Z. Wang, Y. Shi, Y.-M. Shen, W. Wang, Z. Chen, J. Xu, J. Cao, J. Mater. Chem. A 2021, 9, 27028–27033;
- 4eP. S. Borchers, M. Strumpf, C. Friebe, I. Nischang, M. D. Hager, J. Elbert, U. S. Schubert, Adv. Energy Mater. 2020, 10, 2001825.
- 5
- 5aG. Adams, R. Hollandsworth, B. Webber, Lockheed Missiles and Space Co., Palo Alto, CA (USA). Lockheed Palo Alto Research Lab 1979;
- 5bK. Gong, X. Ma, K. M. Conforti, K. J. Kuttler, J. B. Grunewald, K. L. Yeager, M. Z. Bazant, S. Gu, Y. Yan, Energy Environ. Sci. 2015, 8, 2941–2945.
- 6J. Mcbreen, J. Electroanal. Chem. Interfacial Electrochem. 1984, 168, 415–432.
- 7X. Li, Y. Yao, C. Liu, X. Jia, J. Jian, B. Guo, S. Lu, W. Qin, Q. Wang, X. Wu, Angew. Chem. Int. Ed. 2023, 62, e202304667.
- 8
- 8aD. G. Kwabi, K. Lin, Y. Ji, E. F. Kerr, M.-A. Goulet, D. De Porcellinis, D. P. Tabor, D. A. Pollack, A. Aspuru-Guzik, R. G. Gordon, M. J. Aziz, Joule 2018, 2, 1894–1906;
- 8bK. Lin, R. Gómez-Bombarelli, E. S. Beh, L. Tong, Q. Chen, A. Valle, A. Aspuru-Guzik, M. J. Aziz, R. G. Gordon, Nat. Energy 2016, 1, 1–8;
- 8cY. Shi, Z. Wang, Y. Yao, W. Wang, Y.-C. Lu, Energy Environ. Sci. 2021, 14, 6329–6337;
- 8dC. Wang, Z. Yang, Y. Wang, P. Zhao, W. Yan, G. Zhu, L. Ma, B. Yu, L. Wang, G. Li, J. Liu, Z. Jin, ACS Energy Lett. 2018, 3, 2404–2409;
- 8eL. Tong, M.-A. Goulet, D. P. Tabor, E. F. Kerr, D. De Porcellinis, E. M. Fell, A. Aspuru-Guzik, R. G. Gordon, M. J. Aziz, ACS Energy Lett. 2019, 4, 1880–1887.
- 9
- 9aG. Wang, H. Zou, Z. Xu, A. Tang, F. Zhong, X. Zhu, C. Qin, M. Ding, W. You, C. Jia, Mater. Today 2022, 28, 101061;
- 9bH. Zou, Z. Xu, L. Xiong, J. Wang, H. Fu, J. Cao, M. Ding, X. Wang, C. Jia, J. Power Sources 2024, 591, 233856;
- 9cS. Yan, S. Huang, H. Xu, L. Li, H. Zou, M. Ding, C. Jia, Q. Wang, ChemSusChem 2023, 16, e202300710.
- 10J. Luo, B. Hu, C. Debruler, Y. Bi, Y. Zhao, B. Yuan, M. Hu, W. Wu, T. L. Liu, Joule 2019, 3, 149–163.
- 11D. Reber, J. R. Thurston, M. Becker, M. P. Marshak, Cell Rep. Phys. Sci. 2023, 4, 101215.
- 12L. Zhi, C. Liao, P. Xu, F. Sun, F. Fan, G. Li, Z. Yuan, X. Li, Angew. Chem. Int. Ed. 2024, 63, e202403607.
- 13Z. Yuan, Y. Duan, T. Liu, H. Zhang, X. Li, iScience 2018, 3, 40–49.
- 14
- 14aH. Chen, Z. Wang, S. Zhang, M. Cheng, F. Chen, Y. Xu, J. Luo, J. Electrochem. Soc. 2021, 168, 110547;
- 14bS. Sreenath, N. K. Sharma, R. K. Nagarale, RSC Adv. 2020, 10, 44824–44833;
- 14cM. Shin, C. Noh, Y. Chung, Y. Kwon, Chem. Eng. J. 2020, 398, 125631;
- 14dK. Lin, Q. Chen, M. R. Gerhardt, L. Tong, L. E. Sang, B. Kim, A. W. Valle, D. Hardee, R. G. Gordon, M. J. Aziz, M. P. Marshak, Science 2015, 349, 1529–1532;
- 14eY. Ji, M. A. Goulet, D. A. Pollack, D. G. Kwabi, S. Jin, D. Porcellinis, E. F. Kerr, R. G. Gordon, M. J. Aziz, Adv. Energy Mater. 2019, 9, 1900039;
- 14fA. Orita, M. G. Verde, M. Sakai, Y. S. Meng, Nat. Commun. 2016, 7, 13230;
- 14gC. Wang, X. Li, B. Yu, Y. Wang, Z. Yang, H. Wang, H. Lin, J. Ma, G. Li, Z. Jin, ACS Energy Lett. 2020, 5, 411–417;
- 14hA. Hollas, X. Wei, V. Murugesan, Z. Nie, B. Li, D. Reed, J. Liu, V. Sprenkle, W. Wang, Nat. Energy 2018, 3, 508–514;
- 14iY. Chen, M. Zhou, Y. Xia, X. Wang, Y. Liu, Y. Yao, H. Zhang, Y. Li, S. Lu, W. Qin, X. Wu, Q. Wang, Joule 2019, 3, 2255–2267;
- 14jM. Gao, S. Huang, F. Zhang, Y. M. Lee, S. Huang, Q. Wang, Mater. Today 2020, 18, 100540;
- 14kS. Guiheneuf, A. Lê, T. Godet-Bar, L. Chancelier, J. M. Fontmorin, D. Floner, F. Geneste, ChemElectroChem 2021, 8, 2526–2533;
- 14lJ. Hu, C. Yuan, L. Zhi, H. Zhang, Z. Yuan, X. Li, Adv. Funct. Mater. 2021, 31, 2102167;
- 14mR. Feng, X. Zhang, V. Murugesan, A. Hollas, Y. Chen, Y. Shao, E. Walter, N. P. N. Wellala, L. Yan, K. M. Rosso, W. Wang, Science 2021, 372, 836–840;
- 14nW. Lee, G. Park, Y. Kwon, Chem. Eng. J. 2020, 386, 123985;
- 14oP. Zuo, C. Ye, Z. Jiao, J. Luo, J. Fang, U. S. Schubert, N. B. McKeown, T. L. Liu, Z. Yang, T. Xu, Nature 2023, 617, 299–305;
- 14pY. Li, Y. Liu, Z. Xu, Z. Yang, Ind. Eng. Chem. Res. 2019, 58, 10707–10712;
- 14qY. Long, Z. Xu, G. Wang, H. Xu, M. Yang, M. Ding, D. Yuan, C. Yan, Q. Sun, M. Liu, C. Jia, iScience 2021, 24, 103157.
- 15
- 15aJ. Hu, M. Yue, H. Zhang, Z. Yuan, X. Li, Angew. Chem. Int. Ed. Engl. 2020, 59, 6715–6719;
- 15bJ.-M. Fontmorin, S. Guihéneuf, P. Bassil, F. Geneste, D. Floner, Electrochem. Commun. 2021, 132, 107148;
- 15cM. Hu, A. P. Wang, J. Luo, Q. Wei, T. L. Liu, Adv. Energy Mater. 2023, 13, 2203762;
- 15dF. Wang, W. Wu, Z. Lu, B. Yuan, Y. Zhao, T. L. Liu, Mater. Today 2021, 21, 100750.
- 16Z. Z. Yuan, Q. Dai, H. M. Zhang, G. J. Hou, X. F. Li, Joule 2022, 6, 884–905.
- 17J. Wang, R. M. Wolf, J. W. Caldwell, P. A. Kollman, D. A. Case, J. Comput. Chem. 2004, 25, 1157–1174.
- 18G. W. T. M. J. Frisch, H. B. Schlegel, G. E. Scuseria, J. R. C. M. A. Robb, G. Scalmani, V. Barone, B. Mennucci, H. N. G. A. Petersson, M. Caricato, X. Li, H. P. Hratchian, J. B. A. F. Izmaylov, G. Zheng, J. L. Sonnenberg, M. Hada, K. T. M. Ehara, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, O. K. Y. Honda, H. Nakai, T. Vreven, J. A. Montgomery Jr., F. O. J. E. Peralta, M. Bearpark, J. J. Heyd, E. Brothers, V. N. S. K. N. Kudin, R. Kobayashi, J. Normand, A. R. K. Raghavachari, J. C. Burant, S. S. Iyengar, J. Tomasi, N. R. M. Cossi, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, C. A. V. Bakken, J. Jaramillo, R. Gomperts, R. E. Stratmann, A. J. A. O. Yazyev, R. Cammi, C. Pomelli, J. W. Ochterski, K. M. R. L. Martin, V. G. Zakrzewski, G. A. Voth, J. J. D. P. Salvador, S. Dapprich, A. D. Daniels, J. B. F. O. Farkas, J. V. Ortiz, J. Cioslowski, D. J. Fox, Gaussian, Inc., Wallingford CT 2009.
- 19L. Martínez, R. Andrade, E. G. Birgin, J. M. Martínez, J. Comput. Chem. 2009, 30, 2157–2164.
- 20D. A. Case, I. Y. Ben-Shalom, S. R. Brozell, D. S. Cerutti, T. E. Cheatham III, V. W. D. Cruzeiro, T. A. Darden, R. E. Duke, D. Ghoreishi, M. K. Gilson, H. Gohlke, A. W. Goetz, D. Greene, R. Harris, N. Homeyer, S. Izadi, A. Kovalenko, T. Kurtzman, T. S. Lee, S. LeGrand, P. Li, C. Lin, J. Liu, T. Luchko, R. Luo, D. J. Mermelstein, K. M. Merz, Y. Miao, G. Monard, C. Nguyen, H. Nguyen, I. Omelyan, A. Onufriev, F. Pan, R. Qi, D. R. Roe, A. Roitberg, C. Sagui, S. Schott-Verdugo, J. Shen, C. L. Simmerling, J. Smith, R. Salomon-Ferrer, J. Swails, R. C. Walker, J. Wang, H. Wei, R. M. Wolf, X. Wu, L. Xiao, D. M. York, P. A. Kollman, AMBER 2018, University of California, San Francisco 2018.
- 21S. Blazquez, C. Vega, J. Chem. Phys. 2022, 156, 154502.
- 22D. Roe, T. Cheatham, J. Chem. Theory Comput. 2013, 9, 3084–3095.
- 23J. Hunter, Comput. Sci. Eng. 2007, 9, 90–95.
- 24R. S. Nicholson, Anal. Chem. 1965, 37, 1351–1355.