A Cytochrome P450 TxtE Model System with Mechanistic and Theoretical Evidence for a Heme Peroxynitrite Active Species
This article relates to:
-
Cover Picture: A Cytochrome P450 TxtE Model System with Mechanistic and Theoretical Evidence for a Heme Peroxynitrite Active Species (Angew. Chem. Int. Ed. 49/2024)
- Pritam Mondal,
- Dhilanka Udukalage,
- Abubaker A. Mohamed,
- Henrik P. H. Wong,
- Sam P. de Visser,
- Gayan B. Wijeratne,
- Volume 63Issue 49Angewandte Chemie International Edition
- First Published online: October 30, 2024
Pritam Mondal
Department of Chemistry and Biochemistry, The University of Alabama, Tuscaloosa, AL 35487 United States
Current address: Department of Chemical Sciences, Indian Institute of Science Education and Research, Mohali, Punjab, 140306 India
Search for more papers by this authorDhilanka Udukalage
Department of Chemistry and Biochemistry, The University of Alabama, Tuscaloosa, AL 35487 United States
Search for more papers by this authorAbubaker A. Mohamed
Manchester Institute of Biotechnology, The University of Manchester, 131 Princess Street, Manchester, M1 7DN United Kingdom
Department of Chemical Engineering, The University of Manchester, Oxford Road, Manchester, M13 9PL United Kingdom
Search for more papers by this authorHenrik P. H. Wong
Manchester Institute of Biotechnology, The University of Manchester, 131 Princess Street, Manchester, M1 7DN United Kingdom
Department of Chemical Engineering, The University of Manchester, Oxford Road, Manchester, M13 9PL United Kingdom
Search for more papers by this authorCorresponding Author
Sam P. de Visser
Manchester Institute of Biotechnology, The University of Manchester, 131 Princess Street, Manchester, M1 7DN United Kingdom
Department of Chemical Engineering, The University of Manchester, Oxford Road, Manchester, M13 9PL United Kingdom
Search for more papers by this authorCorresponding Author
Gayan B. Wijeratne
Department of Chemistry and Biochemistry, The University of Alabama, Tuscaloosa, AL 35487 United States
Search for more papers by this authorPritam Mondal
Department of Chemistry and Biochemistry, The University of Alabama, Tuscaloosa, AL 35487 United States
Current address: Department of Chemical Sciences, Indian Institute of Science Education and Research, Mohali, Punjab, 140306 India
Search for more papers by this authorDhilanka Udukalage
Department of Chemistry and Biochemistry, The University of Alabama, Tuscaloosa, AL 35487 United States
Search for more papers by this authorAbubaker A. Mohamed
Manchester Institute of Biotechnology, The University of Manchester, 131 Princess Street, Manchester, M1 7DN United Kingdom
Department of Chemical Engineering, The University of Manchester, Oxford Road, Manchester, M13 9PL United Kingdom
Search for more papers by this authorHenrik P. H. Wong
Manchester Institute of Biotechnology, The University of Manchester, 131 Princess Street, Manchester, M1 7DN United Kingdom
Department of Chemical Engineering, The University of Manchester, Oxford Road, Manchester, M13 9PL United Kingdom
Search for more papers by this authorCorresponding Author
Sam P. de Visser
Manchester Institute of Biotechnology, The University of Manchester, 131 Princess Street, Manchester, M1 7DN United Kingdom
Department of Chemical Engineering, The University of Manchester, Oxford Road, Manchester, M13 9PL United Kingdom
Search for more papers by this authorCorresponding Author
Gayan B. Wijeratne
Department of Chemistry and Biochemistry, The University of Alabama, Tuscaloosa, AL 35487 United States
Search for more papers by this authorGraphical Abstract
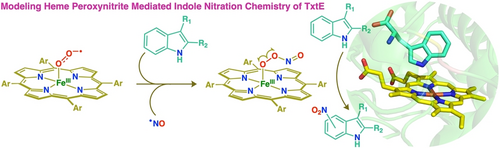
Efficient modelling of the economically impactful enzyme, TxtE, has been probed, in which indole (tryptophan mimic) nitration was observed by a putative heme-peroxynitrite intermediate, resembling one of the prime enzymatic mechanistic proposals. Experimental and theoretical exploration of the mechanism reveal a unique reaction landscape dictated by NO2 radical species.
Abstract
The cytochrome P450 homolog, TxtE, efficiently catalyzes the direct and regioselective aromatic nitration of the indolyl moiety of L-tryptophan to 4-nitro-L-tryptophan, using nitric oxide (NO) and dioxygen (O2) as co-substrates. Pathways for such direct and selective nitration of heteroaromatic motifs present platforms for engineering new nitration biocatalysts for pharmacologically beneficial targets, among a medley of other pivotal industrial applications. Precise mechanistic details concerning this pathway are only weakly understood, albeit a heme iron(III)-peroxynitrite active species has been postulated. To shed light on this unique reaction landscape, we investigated the indole nitration pathway of a series of biomimetic ferric heme superoxide mimics, [(Por)FeIII(O2−⋅)], in the presence of NO. Therein, our model systems gave rise to three distinct nitroindole products, including 4-nitroindole, the product analogous to that obtained with TxtE. Moreover, 15N and 18O isotope labeling studies, along with meticulously designed control experiments lend credence to a heme peroxynitrite active nitrating agent, drawing close similarities to the tryptophan nitration mechanism of TxtE. All organic and inorganic reaction components have been fully characterized using spectroscopic methods. Theoretical investigation into several mechanistic possibilities deem a unique indolyl radical based reaction pathway as the most energetically favorable, products of which, are in excellent agreement with experimental findings.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202409430-sup-0001-misc_information.pdf4.9 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1N. P. Dunham, F. H. Arnold, ACS Catal. 2020, 10, 12239–12255.
- 2A. Spinello, M. Pavlin, L. Casalino, A. Magistrato, Chem. Eur. J. 2018, 24, 10840–10849.
- 3M. Sono, M. P. Roach, E. D. Coulter, J. H. Dawson, Chem. Rev. 1996, 96, 2841–2888.
- 4M. J. Cryle, Metallomics 2011, 3, 323–326.
- 5F. P. Guengerich, The AAPS Journal 2006, 8, 12.
- 6I. A. Pikuleva, M. R. Waterman, J. Biol. Chem. 2013, 288, 17091–17098.
- 7J. D. Rudolf, C.-Y. Chang, M. Ma, B. Shen, Nat. Prod. Rep. 2017, 34, 1141–1172.
- 8D. R. Nelson, Biochim. Biophys. Acta 2018, 1866, 141–154.
- 9P. R. Ortiz de Montellano, Chem. Rev. 2010, 110, 932–948.
- 10A. Greule, J. E. Stok, J. J. De Voss, M. J. Cryle, Nat. Prod. Rep. 2018, 35, 757–791.
- 11Z. Bibi, Nutr. Metab. (Lond.) 2008, 5, 27.
- 12M.-A. Cho, S. Han, Y.-R. Lim, V. Kim, H. Kim, D. Kim, Biomol. Ther. (Seoul) 2019, 27, 127–133.
- 13A. Lampen, U. Christians, F. P. Guengerich, P. B. Watkins, J. C. Kolars, A. Bader, A.-K. Gonschior, H. Dralle, I. Hackbarth, K.-F. Sewing, Drug Metab. Dispos. 1995, 23, 1315–1324.
- 14K.-i. Fujita, Curr. Drug Metab. 2006, 7, 23–37.
- 15K. T. Kivisto, H. K. Kroemer, M. Eichelbaum, Br. J. Clin. Pharmacol. 1995, 40, 523–530.
- 16S. Louka, S. M. Barry, D. J. Heyes, M. Q. E. Mubarak, H. S. Ali, L. M. Alkhalaf, A. W. Munro, N. S. Scrutton, G. L. Challis, S. P. de Visser, J. Am. Chem. Soc. 2020, 142, 15764–15779.
- 17S. M. Barry, J. A. Kers, E. G. Johnson, L. Song, P. R. Aston, B. Patel, S. B. Krasnoff, B. R. Crane, D. M. Gibson, R. Loria, G. L. Challis, Nat. Chem. Biol. 2012, 8, 814–816.
- 18Y. Feng, L. Minjun, X. Chunyan, W. Zhijun, Z. Huan, Y. Min, C. Yaxing, T. Lin, H. Jianhua, PLoS One 2013, 8, e81526.
- 19S. C. Dodani, J. K. B. Cahn, T. Heinisch, S. Brinkmann-Chen, J. A. McIntosh, F. H. Arnold, ChemBioChem 2014, 15, 2259–2267.
- 20H. M. Girvan, A. W. Munro, Curr. Opin. Chem. Biol. 2016, 31, 136–145.
- 21S. C. Dodani, G. Kiss, J. K. B. Cahn, Y. Su, V. S. Pande, F. H. Arnold, Nat. Chem. 2016, 8, 419–425.
- 22R. Zuo, Y. Zhang, C. Jiang, J. C. Hackett, R. Loria, S. D. Bruner, Y. Ding, Sci. Rep. 2017, 7, 842.
- 23G. Jiang, R. Zuo, Y. Zhang, M. M. Powell, P. Zhang, S. M. Hylton, R. Loria, Y. Ding, ACS Catal. 2018, 8, 10761–10768.
- 24R. Zuo, Y. Ding, ACS Synth. Biol. 2019, 8, 857–865.
- 25R. R. King, L. A. Calhoun, Phytochemistry 2009, 70, 833–841.
- 26R. R. King, C. H. Lawrence, L. A. Calhoun, J. Agric. Food Chem. 1992, 40, 834–837.
- 27R. R. King, C. H. Lawrence, M. C. Clark, L. A. Calhoun, J. Chem. Soc. Chem. Commun. 1989, 849–850.
- 28W.-R. d Scheible, B. Fry, A. Kochevenko, D. Schindelasch, L. Zimmerli, S. Somerville, R. Loria, C. R. Somerville, The Plant Cell 2003, 15, 1781–1794.
- 29E. G. Johnson, S. B. Krasnoff, D. R. D. Bignell, W.-C. Chung, T. Tao, R. J. Parry, R. Loria, D. M. Gibson, Mol. Microbiol. 2009, 73, 409–418.
- 30F. G. Healy, M. Wach, S. B. Krasnoff, D. M. Gibson, R. Loria, Mol. Microbiol. 2000, 38, 794–804.
- 31F. G. Healy, S. B. Krasnoff, M. Wach, D. M. Gibson, R. Loria, J. Bacteriol. 2002, 184, 2019–2029.
- 32B. R. Crane, J. Sudhamsu, B. A. Patel, Annu. Rev. Biochem. 2010, 79, 445–470.
- 33J. D. Caranto, Curr. Opin. Chem. Biol. 2019, 49, 130–138.
- 34S. Patterson, S. Wyllie, Trends Parasitol. 2014, 30, 289–298.
- 35R. Aneja, S. N. Vangapandu, M. Lopus, R. Chandra, D. Panda, H. C. Joshi, Mol. Pharmacol. 2006, 69, 1801.
- 36H. Diacon Andreas, R. Dawson, J. du Bois, K. Narunsky, A. Venter, R. Donald Peter, C. van Niekerk, N. Erondu, M. Ginsberg Ann, P. Becker, K. Spigelman Melvin, Antimicrob. Agents Chemother. 2012, 56, 3027–3031.
- 37L. Zhou, G. Stewart, E. Rideau, N. J. Westwood, T. K. Smith, J. Med. Chem. 2013, 56, 796–806.
- 38N. J. Ryan, J. H. Lo, Drugs 2014, 74, 1041–1045.
- 39G. Priotto, S. Kasparian, W. Mutombo, D. Ngouama, S. Ghorashian, U. Arnold, S. Ghabri, E. Baudin, V. Buard, S. Kazadi-Kyanza, The Lancet 2009, 374, 56–64.
- 40R. Saroay, G.-D. Roiban, L. M. Alkhalaf, G. L. Challis, ChemBioChem 2021, 22, 2262–2265.
- 41C. P. Martin, M. Chen, M. F. Martinez, Y. Ding, J. D. Caranto, Biochemistry 2021, 60, 2436–2446.
- 42S. Herold, T. Matsui, Y. Watanabe, J. Am. Chem. Soc. 2001, 123, 4085–4086.
- 43A. Sala, S. Nicolis, R. Roncone, L. Casella, E. Monzani, Eur. J. Biochem. 2004, 271, 2841–2852.
- 44W. Jantschko, P. G. Furtmüller, M. Allegra, M. A. Livrea, C. Jakopitsch, G. Regelsberger, C. Obinger, Arch. Biochem. Biophys. 2002, 398, 12–22.
- 45R. Ricoux, J.-L. Boucher, D. Mansuy, J.-P. Mahy, Eur. J. Biochem. 2001, 268, 3783–3788.
- 46R. Ricoux, E. Girgenti, H. Sauriat-Dorizon, D. Blanchard, J.-P. Mahy, J. Protein Chem. 2002, 21, 473–477.
- 47M. R. Buddha, T. Tao, R. J. Parry, B. R. Crane, J. Biol. Chem. 2004, 279, 49567–49570.
- 48T. Suzuki, H. F. Mower, M. D. Friesen, I. Gilibert, T. Sawa, H. Ohshima, Free Radical Biol. Med. 2004, 37, 671–681.
- 49S. Padmaja, M. S. Ramazenian, P. L. Bounds, W. H. Koppenol, Redox Rep. 1996, 2, 173–177.
- 50B. Alvarez, H. Rubbo, M. Kirk, S. Barnes, B. A. Freeman, R. Radi, Chem. Res. Toxicol. 1996, 9, 390–396.
- 51P. Comba, M. Kerscher, Coord. Chem. Rev. 2009, 253, 564–574.
- 52A. R. McDonald, L. Que, Coord. Chem. Rev. 2013, 257, 414–428.
- 53K. Ray, F. F. Pfaff, B. Wang, W. Nam, J. Am. Chem. Soc. 2014, 136, 13942–13958.
- 54G. Mukherjee, J. K. Satpathy, U. K. Bagha, M. Q. E. Mubarak, C. V. Sastri, S. P. de Visser, ACS Catal. 2021, 9761–9797.
- 55L. Vicens, G. Olivo, M. Costas, ACS Catal. 2020, 10, 8611–8631.
- 56S. M. Adam, G. B. Wijeratne, P. J. Rogler, D. E. Diaz, D. A. Quist, J. J. Liu, K. D. Karlin, Chem. Rev. 2018, 118, 10840–11022.
- 57S. K. Sharma, A. W. Schaefer, H. Lim, H. Matsumura, P. Moënne-Loccoz, B. Hedman, K. O. Hodgson, E. I. Solomon, K. D. Karlin, J. Am. Chem. Soc. 2017, 139, 17421–17430.
- 58P. Wigner, P. Czarny, P. Galecki, T. Sliwinski, Psychiatr. Danubina 2017, 29, 394–400.
- 59F. Peyrot, C. Ducrocq, J. Pineal Res. 2008, 45, 235–246.
- 60P. Mondal, G. B. Wijeratne, J. Am. Chem. Soc. 2020, 142, 1846–1856.
- 61P. Mondal, I. Ishigami, E. F. Gérard, C. Lim, S.-R. Yeh, S. P. de Visser, G. B. Wijeratne, Chem. Sci. 2021, 12, 8872–8883.
- 62P. Mondal, I. Ishigami, S.-R. Yeh, G. B. Wijeratne, Angew. Chem. Int. Ed. 2022, 61, e202211521.
- 63T. S. Kurtikyan, G. G. Martirosyan, M. E. Hakobyan, P. C. Ford, Chem. Commun. 2003, 1706–1707.
- 64P. Mondal, G. B. Tolbert, G. B. Wijeratne, J. Inorg. Biochem. 2022, 226, 111633–111645.
- 65Notably, no spectral evidence for the formation of any reaction intermediates, for example, heme ferric peroxynitrite, could be observed even at −135 °C in 2-methyl-THF solvent; see Figure S4.
- 66M. P. Schopfer, B. Mondal, D.-H. Lee, A. A. N. Sarjeant, K. D. Karlin, J. Am. Chem. Soc. 2009, 131, 11304–11305.
- 67Such a six-coordinate heme complex would encompass a low-spin FeIII center, which spin couples with ⋅NO to result in an overall diamagnetic system.
- 68J. Su, J. T. Groves, Inorg. Chem. 2010, 49, 6317–6329.
- 69
- 69aSimilar nitroindole yields were also observed when 1 equiv of NO(g) was utilized by means of NO(g) solubilized in THF solvent;
- 69bWe note here that N-nitroindoles exhibit impaired stabilities, and therefore their calculated yields could be less than actual reaction yields.
- 70Please see Supporting Information for characterization details on all the nitroindole products. Note that these results should be viewed within the caveat that NO(g) is present in excess under these conditions.
- 71Direct reactivity of peroxynitrite anions with indoles, with and without the presence of ferric heme did not yield any nitroindole products.
- 72Our computational findings suggest that the formation of heme nitrate is highly favorable under these conditions.
- 73N. Lehnert, E. Kim, H. T. Dong, J. B. Harland, A. P. Hunt, E. C. Manickas, K. M. Oakley, J. Pham, G. C. Reed, V. S. Alfaro, Chem. Rev. 2021, 121, 14682–14905.
- 74
- 74aG. Ferrer-Sueta, N. Campolo, M. Trujillo, S. Bartesaghi, S. Carballal, N. Romero, B. Alvarez, R. Radi, Chem. Rev. 2018, 118, 1338–1408;
- 74bC. Batthyány, J. M. Souza, R. Durán, A. Cassina, C. Cerveñansky, R. Radi, Biochemistry 2005, 44, 8038–8046.
- 75J. Lee, J. A. Hunt, J. T. Groves, J. Am. Chem. Soc. 1998, 120, 6053–6061.
- 76J. Lee, J. A. Hunt, J. T. Groves, J. Am. Chem. Soc. 1998, 120, 7493–7501.
- 77P. Astolfi, M. Panagiotaki, C. Rizzoli, L. Greci, Org. Biomol. Chem. 2006, 4, 3282–3290.
- 78B. Blanchard, D. Pompon, C. Ducrocq, J. Pineal Res. 2000, 29, 184–192.
- 79H. E. Gering, X. Li, H. Tang, P. D. Swartz, W.-C. Chang, T. M. Makris, J. Am. Chem. Soc. 2023, 145, 19256–19264.
- 80In support, when indole concentrations are increased up to ten-fold, nitroindole yields are increased only by modest amounts (i.e., 2 a: 10 %; 2 a′: 9 %), and the total yields still remain below 50 %.
- 81S. K. Sharma, P. J. Rogler, K. D. Karlin, J. Porphyrins Phthalocyanines 2015, 19, 352–360.
- 82P. R. Gardner, Scientifica 2012, 2012, 683729.
- 83I. M. Wasser, S. de Vries, P. Moënne-Loccoz, I. Schröder, K. D. Karlin, Chem. Rev. 2002, 102, 1201–1234.
- 84P. Mondal, S. Rajapakse, G. B. Wijeratne, J. Am. Chem. Soc. 2022, 144, 3843–3854.
- 85S. Chen, M. Zhang, R. Su, X. Chen, B. Feng, Y. Yang, J. You, ACS Catal. 2019, 9, 6372–6379.
- 86A. S. Faponle, M. G. Quesne, S. P. de Visser, Chem. Eur. J. 2016, 22, 5478–5483.
- 87C. C. G. Yeh, C. Pierides, G. N. L. Jameson, S. P. de Visser, Chem. Eur. J. 2021, 27, 13793–13806.
- 88A. Wójcik, M. Radoń, T. Borowski, J. Phys. Chem. A 2016, 120, 1261–1274.
- 89J. Xue, J. Lu, W. Lai, Phys. Chem. Chem. Phys. 2019, 21, 9957–9968.
- 90C. C. G. Yeh, S. Ghafoor, J. K. Satpathy, T. Mokkawes, C. V. Sastri, S. P. de Visser, ACS Catal. 2022, 12, 3923–3937.