Adaptive Supramolecular Networks: Emergent Sensing from Complex Systems
Graphical Abstract
Abstract
Molecular differentiation by supramolecular sensors is typically achieved through sensor arrays, relying on the pattern recognition responses of large panels of isolated sensing elements. Here we report a new one-pot systems chemistry approach to differential sensing in biological solutions. We constructed an adaptive network of three cross-assembling sensor elements with diverse analyte-binding and photophysical properties. This robust sensing approach exploits complex interconnected sensor-sensor and sensor-analyte equilibria, producing emergent supramolecular and photophysical responses unique to each analyte. We characterize the basic mechanisms by which an adaptive network responds to analytes. The inherently data-rich responses of an adaptive network discriminate among very closely related proteins and protein mixtures without relying on designed protein recognition elements. We show that a single adaptive sensing solution provides better analyte discrimination using fewer response observations than a sensor array built from the same components. We also show the network's ability to adapt and respond to changing biological solutions over time.
In systems chemistry, networks of interacting and reacting molecules respond to changing conditions in complex ways. Complex systems can generate emergent properties that are hard or impossible to predict a priori. Some examples of system complexity explore kinetically trapped metastable self-assemblies,1 and out-of-equilibrium dissipative self-assemblies.2 Supramolecular complexity can also be introduced by building a system in which multiple building blocks simultaneously interact with each other and establish a complex web of interconnected equilibria.3
Complex molecular systems have been used to create multi-responsive sensors. Different than analytical methods that rely on high-specificity binding elements (e.g. antibodies) to detect a single bioactive analyte,4 multi-responsive chemical sensors detect many target analytes and help characterize mixtures of analytes.5 In this context, “multi-responsive” can mean both “responding to multiple analytes” and also “producing varying kinds of optical responses to any given analyte.” Supramolecular sensing that involves selectively binding individual analytes is routinely done through displacement assays, often utilizing an external host-reporter dye.6 Several host-type sensors are typically deployed in an array to generate characteristic analyte-patterned responses for discrimination (Figure 1a).7 This approach often requires large arrays of sensor elements used in isolation from each other (i.e. in different wells in a microwell plate). Alternative approaches of small molecule dynamic combinatorial libraries,8 and cross-reactive sensors have shown advantages in differential sensing.9 The scope of biological targets and complexity of macrocyclic host-type sensors is being pushed with recent advances deploying covalently attached macrocycle-sensors,10 macrocyclic arrays to identify small modifications in DNA,11 and new strategies of adjusting host-sensor combinations and solution environments to discriminate cell lines and complex protein mixtures.12
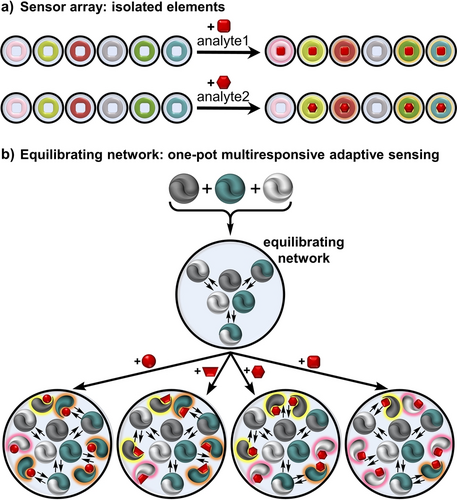
Complex systems offer data-rich outputs in a single solution. a) Array-based sensing requires a panel of individual sensor-analyte response patterns to achieve discrimination. b) We propose a one-pot adaptive sensing network, combining structurally and photochemically diverse sensors to produce fingerprint analyte responses within a single solution.
Here we report a one-pot systems chemistry sensing approach of interacting and reacting sensors that provide data-rich outputs in a single solution. The key to unlocking a functional adaptive network of sensors is using responsive sensor elements that contain homo- and hetero-assembly interactions, paired with a range of analyte binding properties. The reported output is influenced by a complex interconnected web of sensor-sensor and sensor-analyte equilibria (Figure 1b). Slight shifts in this network equilibrium position upon analyte binding are expressed through multi-responsive outputs from more than one mutually interacting sensor, even from sensor elements that might not directly bind the analyte. In this complex system, supramolecular emergent properties arise from the unpredictable adaptations of the network's overall equilibrium position. Photophysical emergent properties arise from the complex, unpredictable ground-state and excited-state interactions of heterodimeric sensors juxtaposing different chromophores. Unlike sensor arrays, the adaptive network of sensors operates within a single solution.
To explore this concept, we chose DimerDyes—a family of dimerizing calixarenes containing an integrated fluorophore.13 DimerDyes operate by disassembly-driven sensing mechanisms. In the absence of analyte, individual DimerDyes self-assemble into homodimers, where two dyes stack in an antiparallel arrangement and the dyes’ emission is quenched. The addition of a good analyte out-competes dimerization to form a host-analyte complex, resulting in a turn-on fluorescence response (Figure 2a). We postulated that a mixture of DimerDyes would self-assemble into homo- and hetero-dimers, thus creating a complex network of assembled sensors. Here, we report an adaptive network that arises from three sensors combining different binding and optical properties in a single solution. Exploiting the combination of emergent supramolecular and photophysical properties, we show that sensing capabilities arise from an adaptive network of mutually assembling sensors, which prove to be more powerful than a sensor array built from the same components.
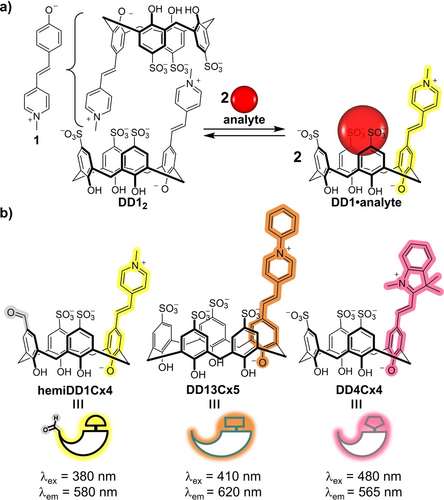
Structurally and photophysically diverse DimerDyes for cross-reactive one-pot sensing. a) Previously reported DimerDye1 (DD1) integrates a merocyanine chromophore (1) into the calix[4]arene scaffold. DD1 operates by a disassembly driven turn-on fluorescence sensing mechanism when an analyte binds the calixarene cavity.13a b) Chemical structures of DimerDye sensors used in this work vary in optical properties, cavity size and anionic charge.
Mixing three DimerDyes containing diverse photochemical properties and structures creates a complex, functional sensing network. We selected three merocyanine dyes—N-methyl pyridinium, N-phenyl pyridinium and indolinium—to span a range of absorbance and emission wavelengths and to encourage new photophysical properties to emerge from dye-dye interactions. To diversify analyte binding affinities, these fluorophores were integrated into calix[4]arene and calix[5]arene scaffolds, varying the upper-rim sulfonate anionic charge and hydrophobic cavity size (Figure 2b). Novel hemiDD1Cx4 was synthesized through monosubstitution of a calix[4]arene dialdehyde (Scheme S1). Novel DD13Cx5 was synthesized via aldehyde-functionalized calix[5]arene (Scheme S2). And previously reported DD4Cx4 was synthesized through aldehyde-functionalized calix[4]arene (Scheme S3).13b These isolated DimerDye sensors—hemiDD1Cx4, DD4Cx4 and DD13Cx5—exhibit characteristic signs of homodimerization in aqueous solution, observed by 1H NMR and fluorescence studies of each isolated sensor (Supporting Information). Combining these three sensors in a one-pot mixture of homo- and hetero-assembled sensors creates a DimerDyeNetwork (DDNetwork), an adaptive network of self-assembling sensors with varied guest-binding and photophysical properties.
The DDNetwork contains both homodimers and heterodimers equilibrating in aqueous solution. If each calixarene in the mixture participated exclusively in homodimers (i.e. if the system underwent narcissistic self-sorting),14 then the absorbance of the DDNetwork would equal the sum of its components studied individually. Instead, comparison of the DDNetwork's absorbance spectrum to the mathematically added spectra of the individual sensors shows strong differences in the absorbance profile. This supports that hetero-aggregates form in the DDNetwork (Figure 3b, S13). In the absence of analyte, the DDNetwork maintains an overall quenched fluorescent state, similar to the individual sensors. The different absorbance properties and non-emissive state of the DDNetwork indicate that heterodimer formation occurs in solution. The presence of both homodimers and heterodimers in equilibrium is independently supported by 1H NMR. When hemiDD1Cx4 and DD4Cx4 are combined, new resonances alongside those of each homodimer demonstrate the presence of hetero-aggregates (Figure S12). Data-rich outputs are obtained by observations at different wavelengths when an analyte is added to a single solution of the adaptive network. DimerDye sensors hemiDD1Cx4, DD4Cx4 and DD13Cx5 mixed in an equimolar ratio (1 : 1 : 1) have a certain distribution of homodimers and heterodimers. As the ratio of one DimerDye is increased in the network, the equilibrium shifts towards more homodimer, demonstrating that the network equilibrium responds as expected to changing concentrations (Figure S14). Upon the addition of choline, a promiscuous analyte that binds all DimerDyes to some extent, the DDNetwork is perturbed. This is evidenced by changes in absorbance and turn-on fluorescence responses within the network (Figure 3c). When the DDNetwork is saturated with excess choline the equilibrium position is pushed entirely toward choline-bound monomers (Figure S15). Under this extreme saturating condition, the network should not contain any homo- or heterodimers and the spectra should resemble the data arising from monomeric host–guest complexes. This is observed by the absorbance response of the DDNetwork to 200 eq. choline closely resembling the mathematically added spectra of the isolated DimerDyes under the same saturating conditions (Figure S15d). At sub-saturating amounts of analyte, the DDNetwork includes a mixture of self-assembled homodimers, heterodimers and analyte-bound monomers. In principle, the interconnected equilibria of the adaptive network should be highly sensitive to the identity of a given analyte, even without any specifically designed host–guest recognition elements.
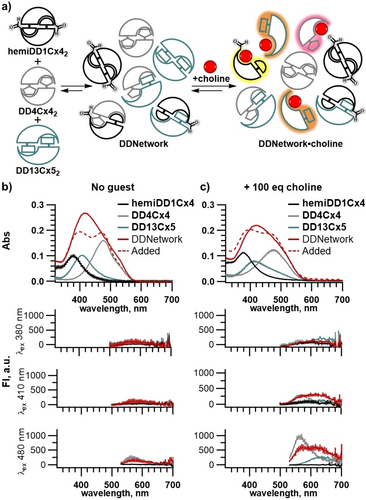
The adaptive DDNetwork behaves differently than the sum of its parts. a) Schematic of DDNetwork assembling into homodimers and heterodimers, followed by analyte binding perturbing the network and inducing a turn-on fluorescence response. b) Absorbance spectra of DDNetwork (solid red line) and the mathematically added spectra (dashed red line) of the individual sensors; hemiDD1Cx4 (black line), DD4Cx4 (grey line) and DD13Cx5 (teal line) are not equal, indicating the presence of heterodimers in the DDNetwork. Fluorescence spectra show the DDNetwork maintains minimal or quenched fluorescence. c) Addition of the analyte choline results in an absorbance profile change and a turn-on fluorescence response. Hosts in all samples are present at identical concentrations—[hemiDD1Cx4]=12 μM, [DD4Cx4]=12 μM and [DD13Cx5]=12 μM—either mixed with each other or separate as indicated. All samples in NaH2PO4/Na2HPO4 (10 mM, pH 7.4) in H2O.
We selected a panel of serum albumin proteins as challenging model analytes for discrimination by the adaptive network. Highly conserved amino acid content, surface charge distribution and crystal structure alignment are reported across several species.15 We selected mammalian serum albumins (SAs) ranging from 69 % to 92 % conserved amino acid identity (Human (HSA), Bovine (BSA), Sheep (SSA), Porcine (PSA), Rat (RSA) and Mouse (MSA)) (Figure 4b, S16–S17, Table S1). Highly conserved sequence identity with homologous hydrophobic binding sites,16 makes them extremely challenging for discrimination by supramolecular sensors.
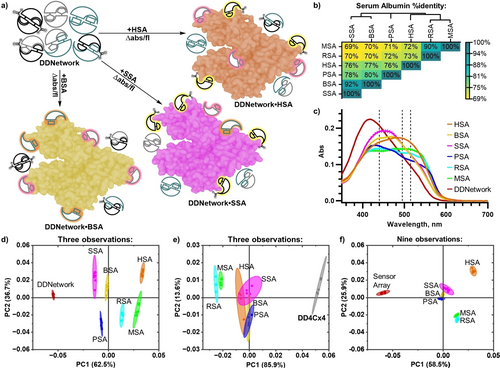
A one-pot adaptive network discriminates highly similar model proteins. a) Schematic of the DDNetwork adaptive interactions with serum albumin proteins resulting in fingerprint sensing patterns. b) Serum albumin sequence %identity is highly conserved across different species. Phylogenetic tree depicts evolutionary commonality between species. c) Unique absorbance profile changes occur upon DDNetwork binding to different serum albumins. Black dashed lines depict absorbance wavelengths used in PCA analysis. PCA score plots of d) DDNetwork e) DD4Cx4 and f) the compiled isolated sensor responses in an array; hemiDD1Cx4, DD4Cx4 and DD13Cx5. PCA analysis was done using absorbance responses at 440, 495 and 515 nm. PCA score plots show each sample set (n=8) enclosed by 95 % confidence ellipses. DimerDyes in all samples are present at identical concentrations—[hemiDD1Cx4]=12 μM, [DD4Cx4]=12 μM and [DD13Cx5]=12 μM—either mixed with each other or separate as indicated, [SA]=32 μM. All samples are in NaH2PO4/Na2HPO4 (10 mM, pH 7.4) in H2O.
A one-pot adaptive network successfully detects and discriminates highly similar proteins. The DDNetwork's complex photophysical responses to these analytes was captured by absorbance and fluorescence spectra at different excitation wavelengths (λex=380, 410, 450, 480 and 510 nm). Each serum albumin protein perturbed the DDNetwork in distinct ways (Figure 4c). Responses were analyzed by Principal Component Analysis (PCA), aiming to minimize the number of observations while achieving the best discrimination. Three absorbance wavelengths (440, 495, 515 nm) achieved complete discrimination of all serum albumin proteins (Figure 4d). Six selected emission responses from the DDNetwork (580 nm (λex=380 nm), 580 nm (λex=410 nm), 620 nm (λex=410 nm), 625 nm (λex=450 nm), 565 nm (λex=480 nm) and 580 nm (λex=510 nm)) achieved discrimination of four serum albumin species from the DDNetwork, with bovine and porcine overlapping (Figure S22).
The adaptive network is more powerful than the equivalent array of individual sensors. The isolated DimerDye sensing responses to serum albumin proteins were measured (Figure S19, S23–S25). The limit of detection (LOD) for HSA and the individual DimerDyes were determined to range from 0.19 to 1.8 μM (Figure S18, Table S2). Notably, isolated DD13Cx5 exhibited the greatest variation in absorbance responses to serum albumin binding. Shifts in λmax up to ≈100 nm (Figure S19d), along with observed 1H NMR conformational flexibility (Figure S6, S31), indicates the calix[5]arene provides distinct conformational changes and sensing properties. HemiDD1Cx4 exhibited the greatest variation in fluorescence responses, possibly due to altered affinities from the upper-rim aldehyde handle (Figure S23). As a direct comparison, the same wavelengths used in PCA analysis of the DDNetwork were applied to the sensing responses of the three isolated DimerDyes in a sensor array. Therefore, sensor array analyses inherently had the advantage of including 3x the number of data observations relative to the one-pot adaptive network. PCA analysis comparison of the compiled isolated sensors in an array (9 absorbance observations, or 18 fluorescence observations) did not achieve complete discrimination with overlapping confidence ellipses of similar proteins (Figure 4f, S20, S26).
The network can adapt quickly to provide real-time analysis of changing biological samples. Trypsin digest experiments of native HSA were conducted to test the DDNetwork's ability to adapt its equilibrium within a changing protein solution over time. Measurable changes in absorbance were observed on the time scale of minutes as HSA underwent proteolysis in both phosphate buffer (pH 7.4) and ammonium bicarbonate buffer (pH 8.0) (Figure S28).
The adaptive network components provide useful sensing readouts despite their undesigned engagement of surface elements. HSA was studied with individual DimerDyes using saturation transfer difference (STD) NMR, which shows how closely engaged ligand protons are to any part of a protein binding partner.17 Positive STD NMR signals were observed for hemiDD1Cx4, DD4Cx4 and DD13Cx5 (Figure S29–S31), providing a general view of the collective complexes formed with HSA. STD intensities for hemiDD1Cx4 (Figure 5b) indicate a predominant complexation geometry where the DimerDye pendant arm and cavity engage the protein and the calixarene lower rim points out towards solvent. To rule out binding in specific ligand-binding pockets, competitive STD NMR was done on hemiDD1Cx4 using saturating amounts of known high affinity competitors that target the primary drug binding sites of HSA.18 Warfarin was used to competitively probe Sudlow site I (Kd HSA≈3 μM),18d, 19 and naproxen was used to competitively probe Sudlow site II (Kd HSA=560 nM).20 The appearance of competitor STD signals was observed in both cases and little to no reduction in the STD signals of hemiDD1Cx4 was observed (Figure S32–35). This data indicates DimerDyes engage serum albumin proteins through surface binding interactions and do not target the primary ligand-binding sites. These results align with crystallography reports of sulfonatocalix[4]arenes binding different proteins at charged surface lysine and arginine residues with multi complexation interactions.21
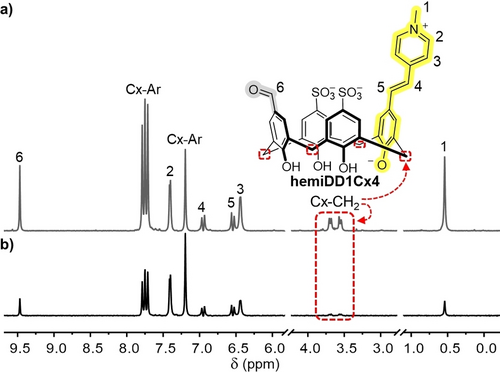
HemiDD1Cx4 binds HSA via pendant arm and upper rim interactions. a) Reference NMR and b) STD NMR of hemiDD1Cx4 (1.2 mM) with HSA (20 μM). Red box indicates lower rim methylene protons have the least intense STD signal. Sample in NaH2PO4/Na2HPO4 (50 mM, pD 7.4) in 90 % H2O/10 % D2O.
Adaptive network sensing can be applied to the discrimination of real-world complex protein mixtures. To further investigate the networks sensing capabilities we extracted protein mixtures from fish tissue samples of cod, halibut, rockfish and sole (Figure 6a). DDNetwork sensing produced variable absorbance and fluorescence responses. These results used in PCA analysis achieve discrimination of extracted protein mixtures from the different white fish varieties (Figure 6, S27). These results further demonstrate the ability of the adaptive network to operate in complex mixtures and illustrates its potential use in applications of tissue classification, food fraud and quality control.
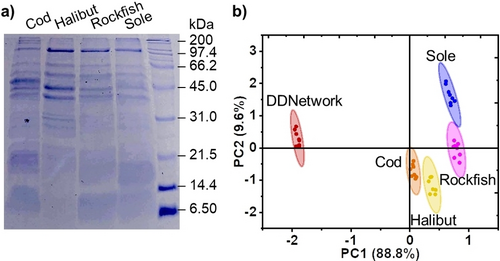
DDNetwork discriminates fish varieties from extracted protein mixtures. a) Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) shows extracted protein mixtures obtained from cod, halibut, rockfish and sole tissue samples. b) PCA score plots show each sample set (n=8) enclosed by 95 % confidence ellipses. Samples contain DDNetwork ([hemiDD1Cx4]=12 μM, [DD4Cx4]=12 μM and [DD13Cx5]=12 μM) and [protein extract]=1 mg/mL. All samples are in NaH2PO4/Na2HPO4 (10 mM, pH 7.4).
Interacting and reacting sensors within a single system provide new kinds of data-rich outputs. The overall emergent profile changes in absorbance and fluorescence produced by the adaptive network provide more powerful tools for discrimination than increased/decreased signal intensities that arise from individual sensors. With the addition of a simple small molecule analyte, a total of 9 possible self-assemblies can occur (3 homodimers, 3 heterodimers and 3 analyte-bound monomers). When analytes become more complex in size and structure, there is an increased number of binding events, modes and equilibria. Varying host-host, host-analyte affinities, and induced host conformational changes are reflected in the range of optical outputs from the adaptive network, proving to be richer in information than the same sensors deployed as an array.
This work illustrates a complex supramolecular system that can be easily built by mixing components. Adaptive systems that embrace promiscuous sensing can be harnessed to achieve challenging sensing tasks. This approach could be applied in any system in which sensor-sensor and sensor-guest interactions combine to create networked complexity.
Supporting Information
The authors have cited additional references within the Supporting Information.22-28, 29-33
Acknowledgments
The authors thank the Natural Sciences and Engineering Research Council of Canada (NSERC, RGPIN-2019-04806) for financial support. A.J.S. thanks NSERC for the Canadian Graduate Scholarship-Doctoral (CGS-D). We thank Christopher Barr for expert assistance in STD NMR and Dr. Cornelia Bohne for helpful discussion. We also thank CAMTEC for the use of shared facilities.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.