High-Density Polyethylene with In-Chain Photolyzable and Hydrolyzable Groups Enabling Recycling and Degradation
Graphical Abstract
Post-polymerization Baeyer–Villiger oxidation of polyethylenes bearing in-chain keto groups (keto-PE) yields mixed keto-ester-PE, which combines photodegradability of the in-chain ketones with the in-chain esters as chemical access point for solvolysis, while the basic materials properties of HDPE are fully retained.
Abstract
Polyethylenes endowed with low densities of in-chain hydrolyzable and photocleavable groups can improve their circularity and potentially reduce their environmental persistency. We show with model polymers derived from acyclic diene metathesis polymerization that the simultaneous presence of both groups has no adverse effect on the polyethylene crystal structure and thermal properties. Post-polymerization Baeyer–Villiger oxidation of keto-polyethylenes from non-alternating catalytic ethylene-CO chain growth copolymerization yield high molecular weight in-chain keto-ester polyethylenes (Mn≈50.000 g mol−1). Oxidation can proceed without chain scission and consequently the desirable materials properties of HDPE are retained. At the same time we demonstrate the suitability of the in-chain ester groups for chemical recycling by methanolysis, and show that photolytic degradation by extended exposure to simulated sunlight occurs via the keto groups.
Plastics are essential components of practically any modern technology. As a downside, persistent plastic waste is found accumulated throughout the world in the environment.1, 2 A better waste management and recycling,3-8 in conjunction with a non-persistent nature of the materials as a backstop is necessary.
For polyethylene, the largest scale synthetically produced plastic,9 the chemical inertness of its hydrocarbon chain hinders both chemical recycling and biodegradation. As a possible solution, the introduction of low densities of in-chain ester groups does not compromise the polyethylene crystal structure and materials properties, but endows the HDPE-like materials with closed loop recyclability and biodegradability.10, 11
While these materials are generated by polycondensation of long-chain difunctional monomers, it would be desirable to also endow polyethylenes from catalytic insertion chain growth with in-chain ester groups. The incorporation of carbon dioxide during ethylene chain growth could theoretically afford such materials. While the reaction has been suggested to be thermodynamically feasible for the relatively low carboxyl group incorporations desired for the above purpose,12-14 it has never been realized. The expected intermediates lack the necessary reactivity, particularly a metal carboxylate formed in a carbon dioxide incorporation event is not prone to further olefin insertion. An alternative to such a direct polymerization is a post-polymerization generation of in-chain ester groups by oxidation of in-chain ketones, as reported by Nozaki for alternating propylene-CO copolymers.15, 16 An access to HDPE materials with in-chain ketones was recently enabled by the long-sought non-alternating copolymerization of ethylene with carbon monoxide, such keto-groups enabling photodegradation to remediate environmental pollution by mismanaged plastics.17, 18
We now report linear polyethylenes with in-chain ester and keto groups, and show how these impact the materials properties and degradability of these HDPE-like materials.
Thermal properties are essential to plastics′ application and processing. In-chain ester as well as keto groups are capable of accommodating in the polyethylene crystal. The resulting energy penalty, which reflects in a reduced melting point, is partially offset by an arrangement of the polar groups into layers in the hydrocarbon crystal.19 This offset correlates with the functional groups′ dipol moment, and consequently keto groups hardly disturb the melting point vs. unfunctionalized polyethylene,20 whereas for ester groups a more pronounced decrease of the melting point is observed.21, 22 In view of these underlying principles, the question arises whether a simultaneous presence of in-chain keto and ester groups will disturb the arrangement that beneficially limits the decrease in melting point vs. HDPE in neat keto-PEs or ester-PEs. To address this, models of mixed keto-ester-PEs with variable functional group densities were generated by ADMET copolymerization of tricosa-1,22-dien-12-one and undec-10-en-1-yl undec-10-enoate, followed by exhaustive hydrogenation (cf. the Supporting Information for experimental details, Table S2–S3). Melting temperatures determined by differential scanning calorimetry (DSC) of these materials with an equimolar amount of ester and keto groups correlate linearly with the functional group density, in line with a Sanchez-Eby inclusion model (Figure 1).23
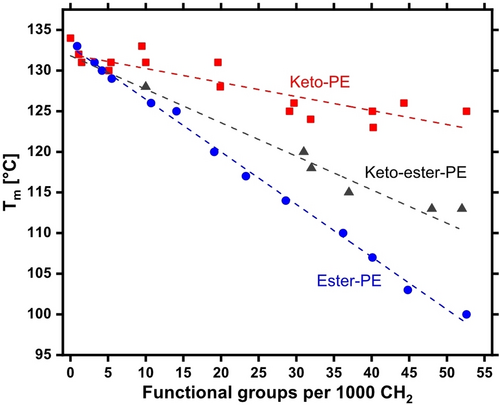
The melting point depression is given by Tm=(132–A×XF) °C with A=423 (XF=number of in-chain functional groups per 1000 methylenes). This is intermittent to neat keto-PEs (A=191)20 and ester-PEs (A=683),21 and agrees with the average of these values within experimental error. That is, no adverse effect of the simultaneous presence of ester and keto groups on thermal properties is observed but rather their contributions are additive, and the data allows for prediction of melting points of targeted compositions.
While the ADMET materials are useful models, their molecular weights are limited due to the step growth nature of the method and also the monomer accessibility is limited in scale. A process that aligns more with industrial chain growth methods is desirable. To obtain high molecular weight keto-PEs, we employed the recently reported Ni(II)-catalyzed nonalternating copolymerization of ethylene with carbon monoxide.17 The resulting materials were subjected to Baeyer–Villiger oxidation (Scheme 1). Virtually complete conversion to in-chain ester groups could be achieved (cf. Figure S11), though we focused on materials that contain residual keto groups as the presence of both types of in-chain groups was targeted (Figure 2). Notably, the oxidation proceeded with little or no chain scission, thus yielding satisfactory high molecular weight keto-ester-PEs (e.g. Mn 48.000 g mol−1, Mw 77.000 g mol−1, with 64 % conversion of initial 1.3 % keto groups. Cf. the Supporting Information for details of oxidation experiments and Table S4 for results of Baeyer–Villiger oxidations). Tensile tests of the obtained keto-ester-PE materials show excellent ductile properties (Et=700 MPa, σy=20.7 MPa, ϵtb=1065 %, cf. Figure S29).
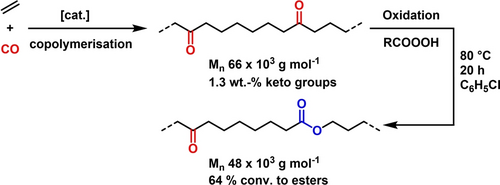
Generation of in-chain ester groups via non-alternating ethylene-CO copolymerization and postpolymerization Baeyer–Villiger oxidation (R=m-ClC6H4). Data from Table S1, entry 4 and Table S4, entry 4.
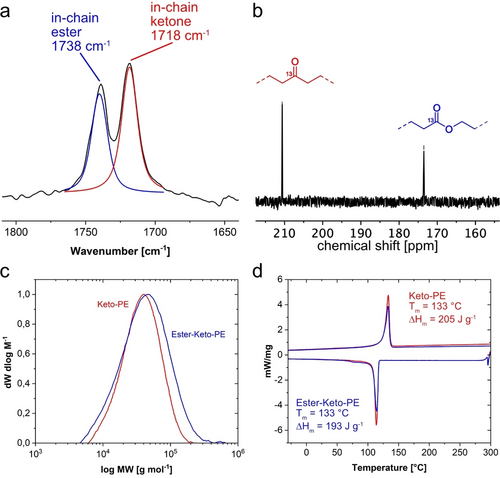
Characterization data of keto-ester-PE from non-alternating ethylene-CO copolymerization and postpolymerization Baeyer–Villiger oxidation (Table S4, entry 1). a: ATR-IR spectrum with band deconvolution showing in-chain ester groups (blue) and in-chain keto groups (red). b: 13C NMR spectroscopy (101 MHz, 373 K, C2D2Cl4) of a 13CO labelled keto-ester-PE (Table S4, entry 2). c: SEC traces of polymers prior (red) and after Baeyer–Villiger oxidation (blue). d: DSC traces of keto-PE (red) and keto-ester-PE (blue) obtained from oxidation.
IR spectroscopic analysis of the polymers after Baeyer–Villiger oxidation reveals the formation of newly formed ester groups, along with residual ketones (Figure 2a). This is confirmed by 13C NMR spectroscopy, facilitated by employing 13CO in the copolymerization, which further underlines the linear nature of these polymers. Notably, backbone −CH2− oxidation to hydroxyl- or further keto-groups was negligible with the employed oxidation procedure and selective transformation of the original in-chain ketones incorporated during ethylene/CO copolymerization was achieved. This is particularly visible in ATR-IR spectroscopy of 13CO labelled keto-ester-PEs by distinguishing the 13C vs. 12C resonances (cf. Figure S13).
As keto-PEs with higher CO contents also contain a minor portion of two closely spaced keto-groups −C(O)CH2CH2C(O)− in the polyethylene chain, this is also reflected in the respective keto-ester-PEs obtained from Baeyer–Villiger reaction. In addition to the prevailing isolated in-chain esters, all other expected oxidation products of the double carbonyl motifs are detected in 13C NMR spectroscopy (cf. Figures S20 and S21). Notably, no preferred conversion or undesired side reactions like promoted chain scission at these motifs were evident.
As expected from the correlations outlined above, the melting point is hardly affected by the oxidation at the functional group densities of interest, and the resulting keto-ester-PEs melting points are essentially similar to HDPE (cf. Figure 2, d and Tables S1 and S4).
Keto-PEs from chain growth copolymerization have been demonstrated to be amenable to photodegradation that reduces their environmental persistency.17, 18 To probe the degradability of keto-ester-PEs and the contribution of the different functional groups, samples with systematically varied composition (from ADMET/hydrogenation) were also exposed to simulated sunlight (cf. Supporting Information for experimental details). SEC revealed a substantial reduction of molecular weight after an exposure for four weeks at a light intensity corresponding to ca. nine months sunlight exposure in Central Europe (cf. Figures S23–S25). The molecular weight reduction correlates essentially to the keto content, as might be expected given that esters are much less prone to Norrish type cleavage reactions.24-26 Photolytic degradation experiments with a higher molecular weight keto-PE from catalytic copolymerization and the respective keto-ester-PE, obtained by Baeyer–Villiger oxidation, showed virtually the same behavior as observed for the ADMET-model polymers (cf. Figure S26–28).
The ester groups, on the other hand, allow for chain cleavage by methanolysis. Methanolysis has been demonstrated to be viable for closed-loop recycling of HDPE-like long-chain polyesters from polycondensation,10 and for polyethylenes with a lower density of ester groups, obtained from (waste) HDPE by a sequence comprising catalytic dehydrogenation, acrylate cross metathesis, and saturation.27 Indeed, acidic methanolysis of our keto-ester-PEs from catalytic chain growth/oxidation yields in-chain keto-functionalized polymers with alcohol and methyl ester chain-ends (cf. Figure S30). This provides a potential combination of the benefits offered by photodegradable in-chain ketones with the chemically cleavable ester-groups.
In summary, high molecular weight polyethylene materials featuring in-chain ester groups are accessible from non-alternating ethylene-carbon monoxide copolymerization and subsequent post-polymerization oxidation. The simultaneous presence of ester and keto-groups has no adverse effect on solid structures which, like mechanical properties, remain HDPE-like. The functional groups′ impact on thermal properties are an additive of those observed in neat keto-PEs and ester-PEs with low densities of functional groups. The presence of in-chain esters provides opportunities for chemical recycling by solvolysis under mild conditions compared to those of polyethylene recycling to monomer. The oxidation procedure calls for improvements in terms of a more sustainable oxidizing agent as well as substrate concentrations. The relevance of these materials certainly warrants further studies of catalytic oxidations with e.g. in situ generated peracids or other benign oxidizing agents.
Author contributions
J.P.B. performed all experiments with ADMET derived polymers. M.B. performed ethylene-CO copolymerizations. N.K.M. developed and employed the oxidation protocol. J.P.B. and R.H. performed photolytic degradation studies. T.O.M. conducted methanolysis experiments and tensile tests. M.B., N.K.M, T.O.M., and S.M. wrote the manuscript.
Acknowledgments
Funding by the ERC (Advanced Grant DEEPCAT, No. 832480) is gratefully acknowledged. We thank Lars Bolk for DSC and SEC analysis, Simon Schwab for support with methanolysis, Melissa Bürkle for WAXS measurements and Robin Kirsten for general technical support. We further thank Dr. Inigo Göttker-Schnetmann for preparation of the UltraCat metathesis catalyst and Levin Elser for contribution to this work as part of his undergraduate studies. Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.