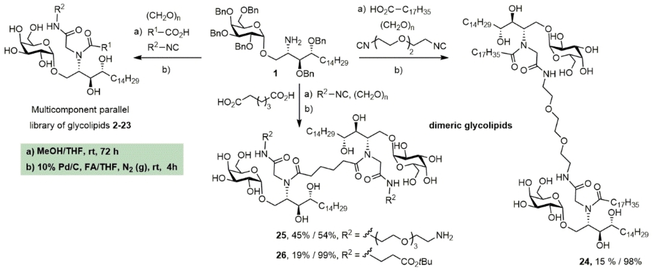
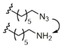
Diversification of a Novel α-Galactosyl Ceramide Hotspot Boosts the Adjuvant Properties in Parenteral and Mucosal Vaccines
Graphical Abstract
A previously underexplored α-GalCer chemical space was discovered and diversified to elicit very high antigen-specific humoral and cellular immune responses. The multicomponent derivatization of the new glycolipid hotspot allowed the incorporation either of extra functionalities that enhanced the adjuvant effect or conjugation handles that are useful for the development of self-adjuvanting vaccines.
Abstract
The development of potent adjuvants is an important step for improving the performance of subunit vaccines. CD1d agonists, such as the prototypical α-galactosyl ceramide (α-GalCer), are of special interest due to their ability to activate iNKT cells and trigger rapid dendritic cell maturation and B-cell activation. Herein, we introduce a novel derivatization hotspot at the α-GalCer skeleton, namely the N-substituent at the amide bond. The multicomponent diversification of this previously unexplored glycolipid chemotype space permitted the introduction of a variety of extra functionalities that can either potentiate the adjuvant properties or serve as handles for further conjugation to antigens toward the development of self-adjuvanting vaccines. This strategy led to the discovery of compounds eliciting enhanced antigen-specific T cell stimulation and a higher antibody response when delivered by either the parenteral or the mucosal route, as compared to a known potent CD1d agonist. Notably, various functionalized α-GalCer analogues showed a more potent adjuvant effect after intranasal immunization than a PEGylated α-GalCer analogue previously optimized for this purpose. Ultimately, this work could open multiple avenues of opportunity for the use of mucosal vaccines against microbial infections.
Introduction
The rapid development of antimicrobial vaccines has proven to be one of the most cost-effective strategies to overcome the challenges posed by emerging infectious diseases. Adjuvants are vaccine components that play an important role owing to their capacity to enhance the immunogenicity of subunit or inactivated vaccines, which comprise the largest number of vaccine formulations.1 In general, either through promoting antigen uptake by antigen presenting cells (APCs) or by activating the innate immune system, adjuvants enhance the adaptive immune response against such rather weak antigens. Unfortunately, the number of clinically validated adjuvants with adaptable profiles to tackle the growing demand of vaccines against emerging viruses and antimicrobial-resistant bacteria and parasites is quite small. Certainly, the challenge lies not only in antigen design and production but in the development of adjuvants either with improved immunostimulation, a novel mode of action, or the capacity to be integrated into self-adjuvanting vaccines.1b, 2 In this latter class of vaccine formulations, the immunogen comprises an antigen that is covalently conjugated—commonly via a cleavable linker—to a potent adjuvant. This is only possible if the adjuvant is suitably functionalized at a position that does not affect its immune-stimulating activity.
In recent years, a subset of innate-like T-lymphocytes known as invariant natural killer T-cells (iNKT) has raised attention due to their characteristic state of partial activation resembling effector memory T cells.3 Strategies stimulating iNKT cells harness and amplify their immunotherapeutic potential, as they play a key role against different infectious diseases such as malaria and HIV, which are proving to be quite resistant to vaccination strategies.4 iNKT cells receptors (αβ-TCRs) recognize lipid and glycolipid ligands presented by the non-polymorphic MHC class-1-like CD1d molecule on APCs. The prototype ligand is α-galactosyl ceramide (α-GalCer, KRN7000), an optimized synthetic derivative of agelasphins.5 α-GalCer-mediated activation leads to rapid CD40-CD40L-dependent dendritic cell maturation and B-cell activation,6 and is characterized by triggering the secretion of a series of cytokines including those related to Th1 (IFN-γ, TNF-α), Th2 (IL-4, IL-5, IL10, IL-13) and Th17 (IL-17A) responses. Th1 cytokines are associated with inflammatory and cellular immune responses and therefore with antitumor,7 antiviral,8 and antibacterial effects,9 while Th2 cytokines are related to humoral immunity—also relevant for antibacterial vaccines—and the amelioration of autoimmune diseases.10 On the other hand, Th17 cytokines enhance host defense against extracellular pathogens.11 While the negative cross-regulation exhibited by the Th1 and Th2 responses might be unwanted when a rather polarized immune response is needed,12 adjuvants triggering a mixed Th1/Th2 response are frequently preferred to cover a wider vaccine spectrum requiring a balanced and potent stimulation of both cellular and humoral responses.13 Polarizing the adaptive immune response has been achieved with different α-GalCer analogues such as OCH—bearing a truncated phytosphingosine chain—, which displays a CD1d low-affinity interaction and biases a Th2 response,14 and 7DW8-5—bearing a terminal p-fluorophenyl group at the acyl chain—, which displays a CD1d high-affinity interaction and biases a Th1 response.15
Most synthetic efforts towards α-GalCer analogues have relied on modifications at the phytosphingosine base,16 the N-acyl lipid chain,17 the glycosidic bond18 and position 6 of the galactosyl moiety19 (Figure 1A). The functionalization of α-GalCer to enable further conjugation to proteins20 (e.g., from SARS-CoV-2) and carbohydrate antigens,21 is considered an alternative vaccine platform to the classic use of carrier proteins. The OH at C-6 of the galactose is the only hydroxyl group not involved in hydrogen bonding in the ternary complex between the glycolipid, the CD1d protein and the T-cell receptor (TCR) of iNKT cells,22 thus it has been the most common site chosen for functionalization en route to immunogenic α-GalCer conjugates.19, 20, 23
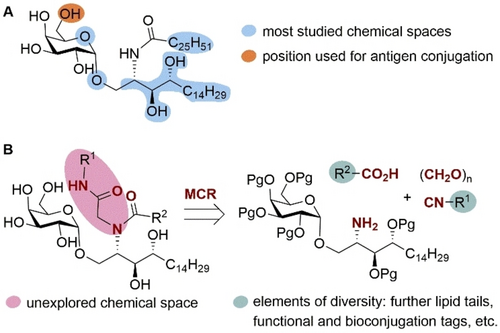
A) Structure of α-GalCer and its most common derivatization sites, including the position used for conjugation to antigens. B) Disconnection strategy for the construction of an α-GalCer multicomponent library covering an unexplored glycolipid space.
Herein we open a new diversification hot-spot at a position that has not been previously derivatized, namely the amide bond connecting the phytosphingosine base and the fatty acid. For this purpose, we introduce a multicomponent reaction (MCR) approach that enables the one-pot construction of a parallel library of α-GalCer derivatives featuring two different diversification sites. Using the power of the Ugi four-component reaction (Ugi-4CR), we populate an unexplored portion of the α-GalCer chemotype space with a wide variety of functionalities assembled in a one-pot reaction, and arising from the carboxylic acid and isonitrile components (Figure 1B). This allows critical adjuvant properties, such as dendritic cell activation and maturation, to be boosted and results in an improved humoral response. Our multicomponent strategy not only exploits a new site for the efficient incorporation of bioconjugation reactive groups without affecting the ligand binding affinity, but also discloses a new family of iNKT agonists featuring dimeric α-GalCer structures (Figure 1C). In the context of vaccine technologies, MCRs have been previously used by our group in the development of antibacterial multivalent glycoconjugates,24 but this is the first time that multicomponent chemistry has been applied in the field of vaccine adjuvants.
Results and Discussion
Multicomponent construction of an adjuvant library
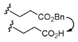
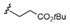
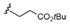
The Ugi-4CR is the condensation of a primary amine, a carboxylic acid, an aldehyde and an isonitrile to form a dipeptide-based (bisamide) scaffold.25 This MCR is very useful for library construction owing to the possibility of varying each of the four components in a combinatorial manner.25 We first built a library of multicomponent α-GalCer adjuvants (MGCAs), varying the nature of the carboxylic acid and isonitrile building blocks while keeping the α-galactosyl phytosphingosine and paraformaldehyde as fixed amino and carbonyl components, respectively (Table 1). With the focus on addressing the effect of the additional amide N-substituent on the adjuvant effect, we decorated the phytoceramide moiety with a third functionality featuring either hydrophobic or hydrophilic characteristics, including lipids, oligoethylene glycol chains, amino acids, charged groups and a fluorescent residue.
|
|||||||||
Entry |
Glycolipid |
R1 |
R2 |
Yield a/b [%] |
Entry |
Glycolipid |
R1 |
R2 |
Yield a/b [%] |
|---|---|---|---|---|---|---|---|---|---|
1 |
2 |
C13H27 |
C12H25 |
84/88 |
12 |
13 |
C23H47 |
|
58/98 |
2 |
3 |
C23H47 |
C18H37 |
65/63 |
13 |
14 |
C25H51 |
|
52/85 |
3 |
4 |
C25H51 |
C18H37 |
56/49 |
14 |
15 |
C25H51 |
|
44/71 |
4 |
5 |
C7H15 |
CH2Ph |
40/95 |
15 |
16 |
C25H51 |
|
51/78 |
5 |
6 |
C25H51 |
CH2Ph |
37/96 |
16 |
17 |
C23H47 |
|
42/63 |
6 |
7 |
C25H51 |
C3H6Ph |
46/97 |
17 |
18 |
C17H35 |
|
35/99 |
7 |
8 |
C11H23 |
c-C6H11 |
54/93 |
18 |
19 |
C25H51 |
|
54/99 |
8 |
9 |
C25H51 |
c-C6H11 |
48/97 |
19 |
20 |
C23H47 |
|
34/89 |
9 |
10 |
|
C14H29 |
30/98 |
20 |
21 |
C25H51 |
|
55/99 |
10 |
11 |
C11H23 |
|
52/94 |
21 |
22 |
C23H47 |
|
72/57 |
11 |
12 |
C15H31 |
|
51/60 |
22 |
23 |
C25H51 |
|
82/82 |
The synthetic methodology consisted of three main parts: the synthesis of the protected α-galactosyl phytosphingosine 1, its multicomponent derivatization and the final global deprotection of the hydroxyl groups by Pd-catalyzed hydrogenation (see the Supporting Information, SI). The Ugi-4CR not only serves as the diversity-generating step, but it also introduces a tertiary amide susceptible to cis/trans isomerization, which can allow two differently populated orientations for the added chains, and therefore have an influence on the binding mode and receptor affinity. Under mild and scalable conditions, we obtained complex and highly diverse glycolipids in moderate to excellent yields after chromatography purification (Table 1). Most functional groups present in the isonitrile and acid substrates were conveniently protected to be either unmasked during the standard benzyl ether removal or further deprotected without affecting the glycolipid structure.
A unique feature of our approach is the possibility of installing not only one but two lipidic tails of variable length at the ceramide moiety. In addition, isonitriles bearing other hydrophobic moieties such as benzyl, 3-phenylpropyl and cyclohexyl groups as well as aromatic amino acids were employed. The reason for this is the presence of aromatic residues in both the invariant Vα24Vβ11 human TCR and lining the CD1d A′ pocket, which are accessible for hydrophobic and π–π stacking interactions.26 The increment in the hydrophobic character could modify not only the mode of interaction and affinity of the iNKT TCR to the α-GalCer−CD1d complex, but also modulate the cytokine profile by enhancing the lipid loading. In compounds 5 and 6 bearing a benzyl amide, the conditions used for the deprotection of the O-benzyl ether did not affect this functionality.
Moreover, we employed more hydrophilic—e.g., oligoethylene glycol-containing—isonitriles in combination with various fatty acids to install a solubilization tag at the ceramide moiety. Previous immunological evidence from a 6“-PEGylated α-GalCer—namely α-GalCerMPEG27—indicated that this PEGylated analogue is suitable for intranasal administration, which is a paradigm of modern vaccinology owing to the relevance of mucosal pathogens such as SAR-CoV-2.28 A third group of Ugi-derived analogues was designed to contain functional groups such as amines, aldehydes or carboxylic acids that could be used for the conjugation of α-GalCer to an antigen for the future development of self-adjuvanting vaccines. A fluorescent α-GalCer derivative was also produced to further facilitate a variety of imaging-based studies (entry 23). Finally, the multicomponent nature of this procedure achieved a feat that had remained elusive so far, namely the synthesis of α-GalCer dimers without touching the galactose unit. This was readily achieved using either a dicarboxylic acid or a diisonitrile, together with 2 equivalents of the other components, thus rendering distinctive scaffolds with different amide connectivity and linkers separating the two glycolipid subunits. Overall, we consider that this library of α-GalCer analogues (MGCAs) stands as one of the largest ever produced in a parallel manner and employing available substrates as the source of structural diversification.
Prediction of ligand affinity
Binding affinities of compounds 2, 3, 4, 7, 8, 9, 10, 11, 12, 14 and 24—chosen as representative of their class within the glycolipid library—were predicted for both the CD1d and the TCR in two sequential steps; first docking the ligands with the CD1d using the VINA algorithm, and then selecting the relevant poses in fixed conformations to calculate the affinity for the TCR using the “Ligand properties” within MOE software. The overall affinity of the ternary complexes was also calculated (Table 1 in the SI).
Docking to the CD1d revealed a binding cavity with a large hydrophobic pocket inside the receptor and a hydrophilic patch on the outer part (Figure 2A–B). While the predicted binding affinities of all ligands for CD1d are similar to that of α-GalCer, compounds 3 and 24 exhibit higher binding affinities for the TCR (Figure 2C). Nevertheless, other relevant poses for these two ligands show comparable binding affinities to those of the rest of the analogues (Table 1 in the SI). For the ternary complexes comprising compounds 2, 3 and 4, which bear hydrophobic chains of different length, we can observe that two chains are accommodated within the hydrophobic pockets of CD1d, while the third chain can potentially interact with the TCR β-chain—of high hydrophobic character—thus adding stabilization to the complexes. By superimposing the docking poses, it is evident that although the sugar is slightly shifted compared to the natural ligand, it certainly conserves the interactions with key residues including Ser27, Asn29, and Arg93 (Figure 2D–E).29 Compounds such as 7, 8 and 9, with extra propyl-phenyl and cyclohexyl-acyl chains, respectively, interact in a similar manner. While the cyclohexyl group presents a favorable hydrophobic interaction within CD1d (Val47, Phe84, Ala144, Val72 Trp131, Phe77, Met87), the propyl-phenyl substituent in compound 7 is long enough to allow the interaction with residue Tyr50 of the TCR β-chain (Figure S120). Interestingly, hydrophilic residues such as the oligoethylene glycol (OEG) in compounds 11 and 12 interact with the more hydrophilic TCR α-chain (Arg93, Ser27, Asp92), but these interactions might also counteract those of the sugar and the CD1d exposed residues with the TCR. In several poses of 11 and 12, the sugar is pushed inside the CD1d while the hydrophilic OEG residue interacts with TCR α-chain residues (Figure S121). During the docking of the dimer 24, one of the sugar heads moves inside the CD1d, while the other head strongly interacts with TCR α-chain residues, thus explaining the enhanced affinity of the glycolipid-CD1d complex towards the TCR (Figure S122).
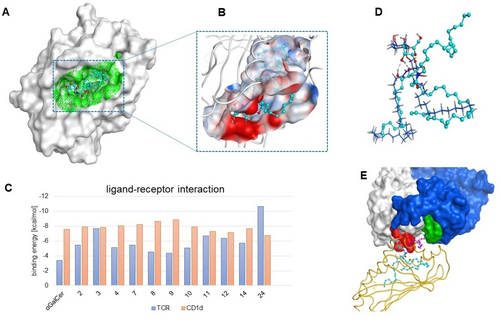
Prediction of ligand affinity by molecular docking. A) Molecular surface of CD1d and its binding pocket (in green) with docked compound 9. B) A close up view of the binding pocket. The receptor is represented in ribbon style, and compound 9 in ball-and-stick form with cyan. Amino acid residues are colored based on their electrostatic surface charge: red for negative, blue for positive, and white for neutral. C) CD1d-ligand and TCR-ligand interactions. Docking of ligands was performed in YASARA software using the VINA algorithm, and binding affinity was calculated by the “ligand properties” feature in MOE software. D) The superposition of the docked pose of compound 3 (bearing an additional lipid chain) with the natural ligand. Natural ligand and compound 3 are represented in blue (line) and cyan (stick and ball), respectively. E) Orientation of compound 3 in the ternary complex. Molecular surface of chains α and β for the TCR receptor are shown in gray and blue, respectively. Key interactions with TCR α-chain and TCR β-chain are shown in red and green, respectively. CD1d is shown in gold (ribbon) and compound 3 in cyan (ball-and-stick). The third chain of compound 3 is shown in purple and interacts with the TCR β-chain.
Evaluation of the adjuvant activity
The co-formulation of the model antigen ovalbumin (OVA) with the MGCAs triggered the activation and maturation of APCs, particularly dendritic cells (DCs) (see the SI). To assess the capacity of the MGCAs to induce antigen-specific T cell stimulation, the novel glycolipids were administered to cultured bone marrow derived cells in an in vitro CFSE assay. Mature antigen presenting DCs were incubated with OVA co-administered either with the potent adjuvant αGalCerMPEG (herein called αGCM)27 or the novel MGCAs. Aiming at determining the effect of these compounds, the pre-treated DCs were subsequently co-incubated with naïve T cells, obtained from transgenic mice (OTI or OTII mice) expressing the OVA-peptide AA257-264 and AA323-339 specific T cell receptor (TCR), respectively. The obtained results revealed that the MGCAs show a higher or comparable capacity, as compared to αGCM, to induce antigen-specific T cell proliferation after interaction with the corresponding OVA peptide presented by mature APCs in the context of MHC-I and MHC-II molecules, respectively. Notably, compound 18 showed a high capacity to induce antigen specific CD4+ and CD8+ T cell proliferation, while compound 20 induced a high antigen specific CD4+ T cell proliferation (see SI, Figure S125). This is very relevant considering that such compounds are α-GalCer analogues bearing functional groups (in a protected form) that are very suitable for conjugation to amino-containing protein or carbohydrate antigens. MGCAs bearing shorter acyl chains as additional functionalization, such as compound 2, showed some degree of toxicity against CD4+ T cells, so they were not included in further studies.
To analyze the T helper polarization in an in vivo CFSE assay, sorted naïve CD4+ T and CD8+ T cells (from OTI or OTII double transgenic mice), were adoptively transferred to normal C57BL/6 mice, and OVA was co-administered with adjuvants such as α-GalCer, αGCM or the MGCAs 3, 4, 6, 7, 9, 13, 14, 15, 16, 19, 21, 23 and 24. Among them, compounds 4, 14, 15 and 21 stimulated significantly higher levels of CD4+ T cells compared to α-GalCer, while compound 4 also showed a significantly enhanced CD4+ T cell stimulation compared to αGCM (Figure S126). In contrast, no enhancement in the proliferative capacity of CD8+ T cells could be observed for any of the molecules. Although not statistically significant for CD8+ T cells, compounds 4, 15 and 21 have a greater capacity for CD8+ T cell proliferation compared to the others (Figure S127).
The dimeric MGCAs presented a very low water solubility, and therefore, were not considered in the present study for further immunological evaluation, but they are of interest for the development of liposomal vaccine formulations.
To further assess the adjuvant effect, we evaluated the response against the model antigen OVA with formulations containing the protein antigen plus MGCAs bearing C24 and C26 acyl chains at the fatty acid position and variable functionalities arising from the isonitrile component (i.e., 3, 4, 6, 7, 9, 13, 14, 15, 16, 19, 21, 23). The immunostimulation effect was compared with the PEGylated analogue αGCM, which was previously reported to have an optimized adjuvant effect with respect to α-GalCer.27 In this context, we aimed at interrogating which MGCAs can improve the antigen-specific humoral response when administered through the parenteral and mucosal routes. Thus, we were interested in MGCAs with an immunopotentiation effect superior or at least similar to that of the αGCM adjuvant, which is already very potent.
Groups of C57BL/6 mice (n=5) were vaccinated by intramuscular (i. m.) or intranasal (i.n.) route three times on days 0, 14 and 28 with OVA protein adjuvanted with αGCM and MGCAs, respectively. As controls, groups of animals (n=5) either non-vaccinated or vaccinated with OVA alone were included. During the course of vaccination, the general behavior, side effects and changes in body weight of vaccinated mice were analyzed. In all groups, neither side effects, changes in behavior nor noticeable problems in body weight were observed (Figure S128).
OVA-specific IgG1 and IgG2c subclass titers were determined in sera samples from vaccinated animals at day 42 (proof of principle animal study). Analysis of the antibody titers in sera induced by i. m. immunization showed an increase in the IgG1 titers (Th2 orientated), and to a lesser degree also in IgG2c (up to 10-fold and 2–4-fold increase, respectively) with respect to those observed in mice receiving OVA alone (Figure 3A). Interestingly, in the i. m. vaccination, various MGCAs stimulated a IgG1/IgG2c profile similar to that of the PEGylated analogue αGCM, indicating in some cases a dominant Th2 humoral response and in others a more balanced Th1/Th2 response. Notably, the strongest antibody response after immunization via the i. m. route was obtained using OVA adjuvanted with MGCA 9. As seen in the molecular docking calculations of 9, the cyclohexyl group at the amide N-substituent (arising from the isonitrile) can favor a strong and long-lasting interaction with the CD1d on DCs based on the additional and favorable hydrophobic interactions. On the other hand, MGCAs 7 and 13 elicited only a marginal humoral response and MGCA 15 showed almost no response (Figure 3A).
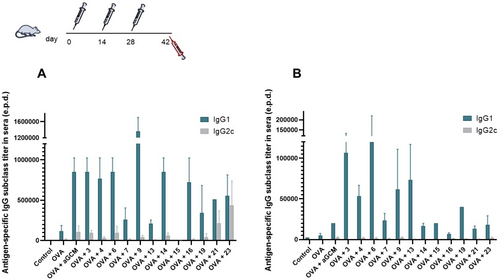
Systemic humoral immune responses induced in sera of vaccinated mice. Animals were immunized on day 0, 14 and 28 with OVA co-administered with and without αGCM and with the novel MGCAs by the A) intramuscular (i. m.) and B) intranasal (i.n.) route. Antigen-specific IgG1 and IgG2c subclass titers in sera were analyzed 14 days after the last immunization. Results are displayed as the average of the last sera dilution (end point dilution, e.p.d), which was 2 times above the mean value of the control background (OD 405 nm).
Very relevant is the case of the α-GalCer analogue 16, which also showed a potent immunostimulation in the i. m. vaccination and bears a free amino group that can be used for conjugation to protein or oligosaccharide antigens. Intriguingly, MGCAs 21 and 23 stimulated a more balanced IgG1/IgG2c ratio, indicating a mixed Th1/Th2 response, with 21 bearing a masked aldehyde functionality that also allows its incorporation into self-adjuvanting immunogens (Figure 3A). This variable antigen-specific immune response depending on the MGCA's functionality is very promising because the immunity can be tuned according to the specific needs for a certain pathogen. Thus, if a stronger antibody response is needed to protect against an extracellular pathogen, MGCAs 3, 4, 6, 9, 14 and 16 could be used, whereas MGCAs 21 and 23 would be beneficial to stimulate an increased Th1 response.
When analyzing the IgG titers elicited by mucosal immunization, a notable enhancement of IgG1 subclass titers (>20-fold increase) was obtained for OVA plus MGCAs compared to the group receiving OVA alone (Figure 3B). This can be considered as a remarkable result, as it is also the fact that many of the novel analogues showed a stronger immunostimulation effect in the i.n. route than the optimized adjuvant αGCM. In this case, the most potent response was obtained for analogue 6, bearing an additional benzyl group at the tertiary amide substituent, which can also favor the CD1d complex stabilization through hydrophobic interactions. Other lipophilic MGCAs like those functionalized with a third lipidic tail (3 and 4) or a short aliphatic or aromatic moiety also stimulated a potent immunity after mucosal vaccination, which in all cases proved to be a dominant Th2 response. Although the hydrophobic stabilization effect seems to be beneficial, as shown in the molecular modelling study, it is worth noting that, for example, analogue 13 bearing a short oligoethylene glycol moiety also showed a notable adjuvant effect that is even superior than that of the 6“-PEGylated α-GalCer αGCM. Based on the immune response resulting from these experiments, it is possible to confirm our hypothesis that the multicomponent N-derivatization of the amide bond is a powerful strategy to produce potent and functionalized α-GalCer adjuvants for a variety of vaccine formulations, which can be tuned either for parenteral or mucosal administration.
Aiming to get deeper insight into the cellular response stimulated by the OVA-MGCAs and OVA-αGCM formulations following i. m. and i.n. immunizations, ELISPOT assays for antigen-specific IFNγ, IL-17, IL-2, IL-10 and IL-4 producing cells and lymphoproliferative assays were performed three weeks after the last dose. In this case, the induction of the highest numbers of IL-4 secreting cells was obtained for the co-administration of OVA-αGCM by i. m. route, which is in agreement with the reported data for this PEGylated adjuvant (Figure 4A).27 The parenteral vaccination with OVA plus the novel MGCAs led—in most cases—to a balanced stimulation of both Th1 and Th2 cytokines secreting cells. Most compounds induced higher numbers of IL-4 secreting cells than OVA alone, and some of them also showed good levels of IL-17 secreting cells, which was not seen after the administration of either OVA alone or OVA-αGCM. Similarly, almost all MGCAs stimulated increased numbers of OVA-specific IFNγ secreting cells with respect to OVA alone (Figure 4A). In addition, OVA co-administered (i. m.) with MGCAs 9, 14 and 23 resulted in the induction of high levels of TNFα/IL-2, IL-2/IFNγ, TNFα/IFNγ or triple positive TNFα/IFNγ/IL-2 CD4+ T cells in response to the OVA protein (Figure S129). In contrast to i. m. immunization, the i.n. administration of OVA-MGCA formulations did not further increase the numbers of IFNγ, IL-2 and IL-4 secreting cells compared to OVA alone (Figure 4B).
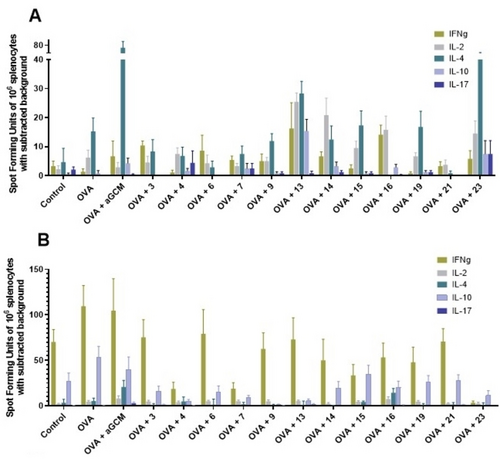
Antigen-specific cytokine-producing cells. Number of cytokine-producing cells derived from spleens of mice vaccinated with different OVA-adjuvant formulations on day 1, 14 and 28 by the A) i. m. or B) i.n. route, as determined by ELISPOT. Results are presented as spot-forming units of 106 antigen-restimulated cells minus the background values from unstimulated cells. The mean from triplicates with two different cell concentrations is shown of control, antigen alone, antigen co-administered with 15 μg αGCM or with novel MGCAs or without adjuvant per dose.
In addition, we analyzed the cytokines secreted in the supernatants of OVA-restimulated splenocytes (Figure S130). These results indicate that, independent of the application route, the novel MGCAs seem to stimulate a mixed Th1/Th2 cellular immune response, as revealed by the enhanced secretion of IFNγ/IL-2 (Th1 cytokines) and IL-5/IL-10/IL-13 (Th2 cytokines). Taken together, we have been able to prove that immunization of mice using the model antigen OVA co-administered with different MGCAs leads to enhanced antigen-specific humoral and cellular immune responses characterized by the predominant production of IgG1 as well as a mixed Th1/Th2 cellular response.
Conclusion
We have shown that the population of a previously unexplored chemical space of α-GalCer using diversity-generating chemistry is a suitable strategy for the discovery of potent adjuvants. Our work illustrates the potential of MCRs in the simultaneous diversification and functionalization of the α-GalCer phytoceramide skeleton and opens a novel derivatization hot-spot for introducing bioconjugation handles toward the development of self-adjuvanting vaccines. Molecular docking studies allowed us to interrogate the binding mode of the novel glycolipids in the TCR-CD1d-ligand ternary complexes and assess how the structural modifications contribute to additional CD1d or TCR α/β-chain interactions. Of particular significance are the markedly high serum IgG subclass titers elicited after mucosal and parenteral administration of OVA plus the novel MGCAs, which were superior to those stimulated by the antigen alone and either comparable (i. m. route) or higher (i.n. route) than those stimulated by the very potent PEGylated analogue αGCM.
Considering the great promise of mucosal vaccines and their recent entrance in the vaccine market,30 the development of efficient mucosal delivery systems and mucosal adjuvants is of high interest. The intranasal administration of different OVA-MGCAs showed a direct effect on the stimulation of CD4+ based immunity, as correlated to the induction of IFNγ/IL-2-secreting cells. Antigen-specific CD4+ T cells enable dendritic cells to optimize antigen presentation and deliver specific cytokine and co-stimulatory signals to other T cells (e.g., CD8+ T), promoting their clonal expansion and differentiation into effector or memory T cells. Our results also demonstrate that depending on the MGCA used as adjuvant, either the humoral or the cellular response can be further increased with some selectivity. Thus, while several MGCAs stimulated increased antibody production, the OVA-specific cellular response was strengthened using other analogues. Further studies are necessary to assess the levels of mucosal immunity elicited by the novel adjuvants.
In summary, our MGCAs constitute promising adjuvants for the development of effective parenteral and mucosal vaccine formulations. While this is the first application of MCRs in the field of vaccine adjuvants, the same strategy can be used to boost the diversification and functionalization of other adjuvant families, including Toll-like receptor agonists, or to make multivalent constructions of different adjuvants toward the development of novel antimicrobial or anticancer vaccines.
Supporting Information
Chemical and immunological methods. NMR and MS data and spectra of the novel adjuvants. Supplementary figures on the antigen-specific humoral and cellular responses elicited by the adjuvants.
Acknowledgments
We are grateful to DAAD, Germany, for PhD fellowships to Y.M., M. Y. and A.V.V., and financial support through the GLACIER project (DAAD 57592717). We thank Hannah Jane Kiely-Collins, University of Cambridge, for helping with grammatical corrections of the manuscript. Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
Some of the authors are inventors of patents including compounds mentioned in this article
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.