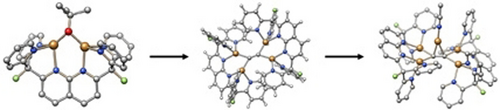
Tetracopper σ-Bound μ-Acetylide and -Diyne Units Stabilized by a Naphthyridine-based Dinucleating Ligand
Graphical Abstract
Reaction of two equivalents of a dicopper(I) tert-butoxide species with an appropriate acetylide synthon allowed the rational synthesis of a molecular tetracopper(I) acetylide, which possesses an unusual μ4-η1:η1:η1:η1 coordination mode. This geometry opens the door to higher nuclearity homo- or heterometallic architectures, as demonstrated by the formation of a pentacopper(I) complex.
Abstract
Reactions of a dicopper(I) tert-butoxide complex with alkynes possessing boryl or silyl capping groups resulted in formation of unprecedented tetracopper(I) μ-acetylide/diyne complexes that were characterized by NMR and UV/Vis spectroscopy, mass spectrometry and single-crystal X-ray diffraction. These compounds possess an unusual μ4-η1:η1:η1:η1 coordination mode for the bridging organic fragment, enforced by the rigid and dinucleating nature of the ligand utilized. Thus, the central π system remains unperturbed and accessible for subsequent reactivity and modification. This has been corroborated by addition of a fifth copper atom, giving rise to a pentacopper acetylide complex. This work may provide a new approach by which metal-metal cooperativity can be exploited in the transformation of acetylide and diyne groups to a variety of substrates, or as a starting point for the controlled synthesis of copper(I) alkyne-containing clusters.
Introduction
Copper(I) complexes bearing alkynyl (−C≡CR, R=alkyl or aryl) ligands have received significant attention due to their interesting photophysical and electronic properties.1 Alkynyl ligands convey characteristic and tunable properties resulting from their various coordination modes, coupled with their linear, rigid structures and electron-rich π system.2 While the chemistry of mono- and polynuclear copper(I) alkynyl species is well documented, copper(I) acetylides (Cu−C≡C−Cu) are scarcely explored,3 despite their crucial role as active species in catalytic ethynylation reactions (Reppe chemistry).4 Indeed, most of the few characterized examples correspond to nanoclusters with nuclearities ranging from Cu24 to Cu92.1, 5 These copper(I) acetylide ensembles are interesting synthetic targets because of their potential applications in electronics and molecular sensing, and in the context of catalysis, and as molecular models for nanostructured and surface copper acetylide entities.1, 5 However, their controlled synthesis is still a challenge, and straightforward synthetic methods are desired.
To date, the lowest nuclearity reported for molecular copper(I) acetylides is four, with only two examples being crystallographically characterized (Figure 1). In 1996, Yam and co-workers described [Cu4(μ-dppm)4(μ4-η1:η1:η2:η2-C≡C)][BF4]2 (dppm=1,1-bis-(diphenylphosphinomethane)), which possesses a C22− fragment located within a rectangular plane defined by the four copper atoms.6 In this structure, the acetylide moiety binds the metal centers in a μ4-η1:η1:η2:η2 fashion, and the complex undergoes two fluxional processes according to NMR and DFT studies: oscillation of the C22− unit inside the rectangle, and flipping of the phosphorus donors above and below the Cu4 plane.7 Similar η1- and η2-binding modes were reported by Mak et al. for [Cu4(μ-Ph2Ppypz)4(μ4-η1:η1:η2:η2-C≡C)][ClO4]2 (Ph2Ppypz=2-(diphenylphosphino-6-pyrazol-1-yl)pyridine), which exhibits a butterfly-shaped Cu4C2 core (rather than a planar structure), thereby maximizing overlap of the two π orbitals of the C22− ligand with metal-based orbitals.8
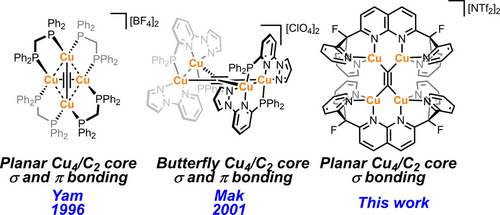
Examples of tetracopper(I) acetylide complexes.
This laboratory has explored the dicopper [(DPFN)Cu2]+ platform containing a rigid, binucleating ligand (DPFN=2,7-bis(fluoro-di(2-pyridyl)methyl)-1,8-naphthyridine),9 and has demonstrated its ability to stabilize reactive, bridging fragments such as boryls,10 nitrenoids,11 or hydrides,12 as well as μ-alkynyl groups.13 In many cases, the Cu2(μ-L) moiety is supported by 3c–2e σ-bonding, with donation of a σ-type lone pair of electrons from the bridging ligand to the low-lying empty 4 s orbitals on the copper atoms.9 In a hypothetical tetracopper(I) acetylide complex supported by two (DPFN)Cu2 fragments, this binding mode should confine the acetylide fragment to σ-type interactions with the metals and leave the π system accessible for further reactivity and modification, in contrast to the tetracopper examples mentioned above. This is expected to allow coordination of additional metal atoms to the acetylide π-orbital, thus providing a starting point for the controlled, bottom-up synthesis of homo- or hetero-nuclear, higher nuclearity carbide nanoclusters.
Here we describe the synthesis and characterization of an unprecedented μ4-η1:η1:η1:η1 tetracopper(I) acetylide molecular complex supported by a 1,8-naphthyridine-based dinucleating ligand. Following a similar synthetic procedure, two additional tetracopper diyne species were obtained. Moreover, the availability of the π electron density to engage in reactivity is demonstrated by addition of a fifth metal equivalent, leading to a pentacopper acetylide complex.
Results and Discussion
Inspiration for this work came from reactivity exhibited by dicopper(I) boryl complexes [(DPFN)Cu2(μ-B(OR)2)][NTf2] (B(OR)2=Bpin (1) or Bcat (2)), which deprotonate terminal aryl alkynes to give the corresponding μ-alkynyl derivatives (Scheme 1).10 These results suggested that related reactions of acetylene, or alkynes with two appropriate leaving groups, could generate a tetracopper(I) acetylide structure. An initial approach involved heating a mixture of boryl complex 1 and 0.5 equivalents of bis(trimethylsilyl)acetylene (BTMSA) in THF at 75 °C for 24 h. However, both starting materials persisted under these reaction conditions (Figure S26). Thus, a complex with a potentially more basic bridging ligand, [(DPFN)Cu2(μ-OtBu)][NTf2] (3), was examined.10
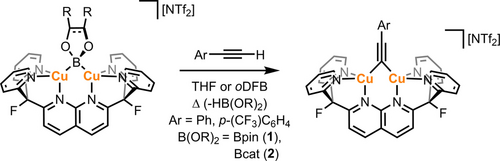
Deprotonation of aromatic terminal alkynes by dicopper(I) boryl complexes 1 and 2. oDFB: 1,2-difluorobenzene.
Heating a THF solution of 3 in the presence of 0.5 equivalents of BTMSA at 70 °C gave [(DPFN)Cu2(μ-C≡CSiMe3)][NTf2] (4) as the main species after 16 h, according to 1H NMR spectroscopy (Figure S27). This was confirmed by independent synthesis of 4 by adding LiC≡CSiMe3 to a THF solution of bridging acetonitrile complex [(DPFN)Cu2(μ-N≡CMe)][NTf2]2 (5) at 25 °C (Scheme 2).9 The reaction mixture was fully converted after 1 h to a new symmetrical species consistent with the previously observed μ-alkynyl complex, according to the 1H NMR spectrum. Diagnostic chemical shifts were observed at 0.27 and −30.5 ppm in the 1H and 29Si-1H HMBC NMR spectra, respectively, indicating the presence of a SiMe3 group. Interestingly, compound 4 was also observed by 1H NMR spectroscopy when the synthetic protocol utilized for the previously reported tetracopper acetylide complexes was attempted, i.e. in situ dilithiation of trimethylsilylacetylene (with two equivalents of n-BuLi) followed by addition of two equivalents of 56, 8 (regardless of the addition order; Figure S28). These results suggest that the formation of 4 is highly favored under the reaction conditions described above.
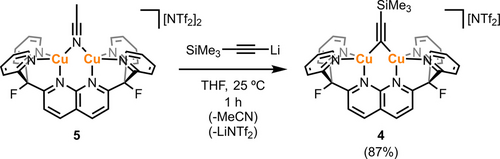
Synthesis of μ-alkynyl complex 4 from species 5. Isolated yield in parenthesis.
Compound 4 was isolated as dark brown crystals suitable for single-crystal X-ray diffraction analysis in 87 % yield by the diffusion of n-pentane into a THF solution at 25 °C. Figure 2 shows the molecular structure of the cationic fragment of 4, revealing the previously mentioned μ2-η1:η1 binding mode with no appreciable π interaction between the copper atoms and the C≡C bond, since the C2−Cu distances (>3.0 Å) are longer than the sum of their covalent radii (2.01 Å).14 Whereas the alkyne C1−C2 distance (1.227(6) Å) of 4 is in good agreement with other dicopper structures containing μ-trimethylsilylalkynyl ligands (1.19–1.24 Å),15 the Cu⋅⋅⋅Cu distance is considerably shorter (2.3822(6) Å vs 2.48–2.64 Å). This is presumably due to the dinucleating character of the DPFN ligand, which enforces closer copper-copper contacts,16 since the Cu⋅⋅⋅Cu distance in 4 is comparable to those observed in other DPFN-supported μ-alkynyl dicopper complexes.13
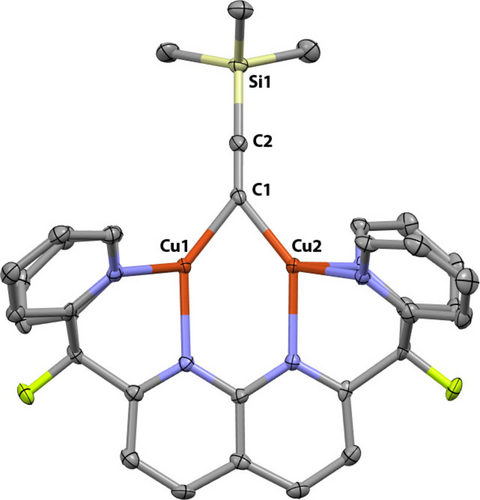
Solid-state molecular structure of the cationic fragment of 4 (50 % probability ellipsoids); H atoms and bis(trifluoromethylsulfonyl)imide (NTf2) anion omitted for clarity.
Efforts were made to utilize complex 4 in synthesis of the tetracopper(I) acetylide [(DPFN)2Cu4(μ4-η1:η1:η1:η1-C≡C)][NTf2]2 (6). For example, attempts to cleave the SiMe3 group with fluoride reagents (e.g. KF/18-crown-6 or tetramethylammonium fluoride) or n-butyllithium resulted in either intractable reaction mixtures or release of the DPFN ligand (a number of other unsuccesful pathways to synthesize 6 can be found in the SI). However, addition of 0.5 equivalents of 1,2-bis(4′,4′,5′,5′-tetramethyl[1′,3′,2′]dioxaborolan-2′-yl)ethyne (B2C2)17 to a THF solution of 3 resulted in formation of a new symmetrical species (Figure 3), according to a 1H NMR spectrum of the reaction mixture. In addition, the expected byproduct tBuOBpin was observed by 1H and 11B{1H} NMR spectroscopy,18 suggesting formation of the desired acetylide species 6. Indeed, single-crystal X-ray diffraction confirmed the identity of this product and the μ4-η1:η1:η1:η1 binding mode, previously unobserved in tetranuclear copper systems. Residual electron density was observed above and below the π system of the acetylide fragment (the Cu2C2Cu2 plane). The subsequent identification of a pentacopper derivative (see below) suggests that this residual peak may correspond to small quantities of a cocrystallized species bearing an additional copper atom. Refinement of the position as a copper atom results in a final occupancy of 4 % per asymmetric unit (8 % per molecule). The cocrystallized impurity was removed from bulk samples of 6 by the addition of one equivalent of a strong σ-donor, IPr (1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene), to the reaction mixture to afford complex 6 as analytically pure dark brown crystals in 74 % yield.
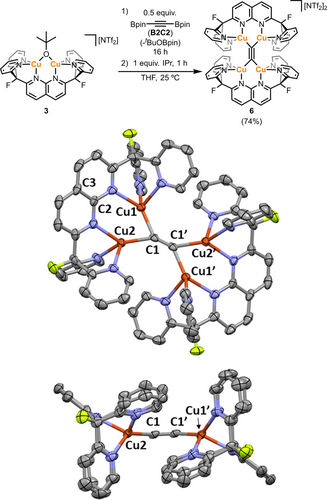
. Top: Synthesis of acetylide complex 6 from 3 (isolated yield in parenthesis). Bottom: Top and side view of the solid-state molecular structure of the cationic fragment of 6 (50 % probability ellipsoids; H atoms, bis(trifluoromethylsulfonyl)imide (NTf2) anions and solvents of crystallization omitted for clarity).
The solid-state structure of 6 (Figure 3) contains a plane defined by the four copper atoms in which the acetylide moiety is contained (the maximum deviation from the plane is defined by the dihedral angle ∠Cu1−C1−C1′−Cu2′=13(1)°). The acetylide fragment exhibits a C1−C1′ distance of 1.242(9) Å, which is slightly longer than that of free acetylene (≈1.20 Å)19 and very similar to that observed for [Cu4(μ-Ph2Ppypz)4(μ4-η1:η1:η2:η2-C≡C)][ClO4]2 (1.259(7) Å).8 The Cu⋅⋅⋅Cu distance (2.4246(8) Å) is slightly longer than that in 4, in spite of both possessing similar ∠Cu−C−Cu angles (75–76°), but it is much shorter than those observed for the examples reported by Yam (>3 Å)6 and Mak (>2.8 Å).8 Curiously, the acetylide fragment is tilted with respect to the naphthyridine backbone (∠C1−C2−C3=162.7(4)°). DFT calculations20 on a single molecule of 6 in the gas phase reproduce this geometry (∠C1−C2−C3=167.3°, see Supporting Information for more information), ruling out crystal packing effects. A space-filling representation of the cationic fragment of 6 (Figure S40) shows that the pyridine groups of opposite DPFN ligands are very close in proximity, and suggests that a planar (instead of a tilted) arrangement would lead to steric clash of the pyridine rings. Moreover, the observed tilted geometry might be additionally favored by C−H/π interactions involving the pyridine side-arms, according to short C⋅⋅⋅H and C⋅⋅⋅C distances (Figure S41).21
The observation of a single species by 1H NMR spectroscopy suggests that the structure identified in the solid state also exists in solution. This is supported by mass spectrometry analysis (Figure S17), which contains a parent ion peak of the expected m/z ratio and isotope distribution pattern. The symmetrical character of complex 6 makes the acetylide C≡C vibration silent by infrared spectroscopy, as corroborated by DFT calculations. Thus, 6 was analyzed by Raman spectroscopy which allowed observation of a band at 1844 cm−1 attributed to the C≡C stretch (Figure S22), 130 cm−1 lower than that of free acetylene (1974 cm−1).22
Regarding the unique η1 binding mode of the acetylide moiety, the DPFN ligand seems to play an instrumental role due to its dinucleating character and its steric bulk, which might prevent π-bonding of the central C22− fragment to the metal atoms (Figure 4). This hypothesis finds support from computational studies, where a relaxed potential energy scan calculation of a truncated version of 6 (NH2 instead of pyridine groups) resulted in η2 coordination to some of the copper atoms (Figures S44–45).23
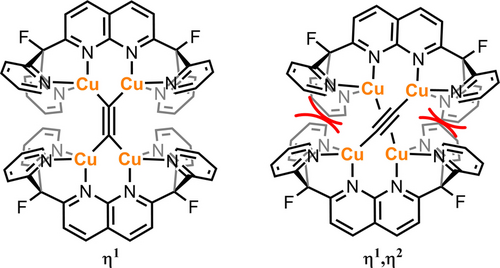
Possible bonding scenarios for the cationic fragment of complex 6.
This laboratory has previously demonstrated that bridging ligands with poor π-accepting character tend to adopt a symmetrical, end-on or η1 coordination mode so that σ-donation to the dicopper core is maximized.9 Indeed, Natural Localized Molecular Orbital (NLMO)24 calculations revealed a 3c–2e bonding mode for both Cu−C−Cu interactions in 6, in which the bridging carbon atoms donate electron density to empty 4 s orbitals on the metals through sp hybrid orbitals of the acetylide group, with no appreciable participation of the electron density located on π-type orbitals (Figures S47–S48). Interestingly, NLMO analysis points to very little to negligible delocalization of electron density between the two terminal dicopper units through the acetylide fragment (Figures S47–S51), which indicates no electronic communication between the metal centers. This phenomenon might be related to the observed binding mode, as electron delocalization between the metal atoms often involves the participation of π-type orbitals.25 The π electrons appear to be energetically accessible, since computational methods characterize the HOMO and HOMO-1 orbitals of 6 as being located on the tetracopper acetylide core as an antibonding combination of copper d orbitals and the π bonds of the C22− fragment (Figure 5).
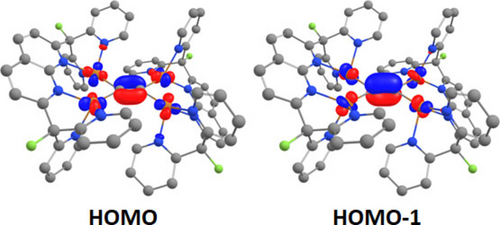
Selected molecular orbitals of complex 6, rendered at an isosurface value of 0.06. H atoms have been omitted for clarity.
Experimentally, the accessibility of the π electrons was observed by addition of 1 equivalent of [Cu(MeCN)4][NTf2] to a THF solution of 6, which cleanly gave pentacopper species 7 in 95 % yield as an analytically pure, dark red-brown solid after work-up (Figure 6). Titration experiments involving addition of Cu+ in 0.5 equivalent increments (up to a total of two equivalents) led to coordination of just one copper atom to the alkyne moiety, as evidenced by analysis of chemical shifts in the 1H NMR spectra (Figure S32). Moreover, X-ray diffraction experiments on single crystals of 7⋅MeCN, grown from a mixture of 6 and two equivalents of [Cu(MeCN)4][PF6],26 provided the pentanuclear structure displayed in Figure 6. One of the main structural features is the presence of a MeCN molecule bound to the fifth metal center. However, results from NMR, IR spectroscopy and combustion analysis of 7 point to a structure without bound MeCN, as depicted in Figure 6 (top). This fifth copper atom does not seem to bind symmetrically in the solid state, as evidenced by the ∠C1−C1′−Cu3 (69.2(2)°) and ∠C1′−C1−Cu3 (75.0(2)°) angles. While DFT reproduces this canted geometry (63.9° and 80.7°, Figure S43), the resonances of the DPFN ligand in the 1H NMR spectrum correspond to those of a symmetrical species.

. Top: Synthesis of pentacopper complex 7 from species 6 (isolated yield in parenthesis). Bottom: Solid-state molecular structure of the cationic fragment of 7⋅MeCN (50 % probability ellipsoids; H atoms, hexafluorophosphate (PF6) anions and solvents of crystallization omitted for clarity).
Coordination of the fifth copper via donation of π electrons from the acetylide fragment elongates the C−C distance to 1.261(3) Å, which is comparable to that described by Mak et al. for their tetracopper system (1.259(7) Å).8 This interaction has been corroborated by NLMO analysis, where donation of the π electron density to the 4 s orbital of the fifth copper atom was observed. Remarkably, no pyramidalization of the carbon atoms in the central C22− fragment was observed in this adduct (omitting Cu3, sum of angles in the tetracopper acetylide plane around C1 or C1′ >359° for both 6 and 7). IR spectroscopy does not reveal a stretch from 1600 to 2900 cm−1, as expected from the very weak intensity predicted by DFT, and Raman measurements were not successful due to the sensitivity of the sample.
The synthetic strategy employed in the synthesis of 6 was investigated for different diynes capped with −SiMe3 groups, to rely on formation of tBuOSiMe3 as a driving force in the transfer of the alkyne fragments to a tetracopper core. To this end, 1,4-bis(trimethylsilyl)-1,3-butadiyne (Si2C4) and 1,6-bis(trimethylsilyl)-1,5-hexadiyne (Si2C6) were utilized as rigid and flexible diyne precursors; respectively, and were treated with 3 (Scheme 3). Contrary to the results observed for BTMSA, addition of 0.5 equivalents of Si2C4 to 3 at 25 °C in THF resulted in the precipitation of a brown solid after 46 hours. Analysis of the supernatant by 1H NMR in THF-H8 revealed no aromatic resonances, indicating that the DPFN-containing species precipitated from the reaction mixture. Dissolving this solid in acetonitrile-d3 resulted in a dark yellow solution with only one set of resonances for the DPFN framework (by 1H NMR analysis), and none for SiMe3 or tBuO groups, pointing to formation of the desired product 8 (Figure S34). This hypothesis was confirmed by X-ray diffraction analysis of single crystals grown by layering iPr2O over a MeCN solution of the complex, which revealed another example of the η1 binding mode previously observed for species 4 and 6 (Figure 7, top).
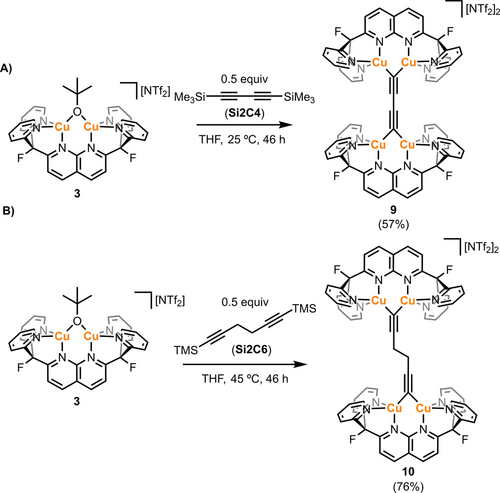
Synthesis of tetracopper complexes 8 (A) and 9 (B) from species 3. Isolated yields in parentheses.
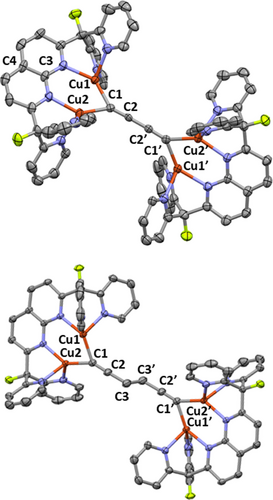
Solid-state molecular structure (50 % probability ellipsoids) of the cationic fragments of 8 (top) and 9 (bottom). H atoms, NTf2 anions and solvents of crystallization omitted for clarity.
The solid-state structure of 8 contains two independent tetracopper cations in the asymmetric unit. Both exhibit similar bonding metrics; however, detailed discussion of the metrical parameters is precluded by issues in resolving the significantly disordered and, in some cases, partially occupied NTf2 anions, of which there are five unique moieties in the asymmetric unit. The solid-state molecular structure of 8 clearly displays linear C42− fragments in each independent molecule; furthermore, alternation between triple- and single-bonds is readily apparent from the C−C bond lengths (C1−C2≈1.22 Å; C2−C2′ ≈1.37 Å), in good agreement with related structures involving group 11 metals.27 Notably, the η1:η1 coordination mode enforced by the DPFN ligand for the C4 group is unprecedented.28 The divergence in reactivity observed between BTMSA and Si2C4 might be due to the extended carbon chain in Si2C4, which prevents the opposing pyridine groups between the DPFN ligands from colliding in the resulting complex 8. Indeed, unlike complex 6, the diyne fragment in the solid-state structure of 8 is not canted with respect to the naphthyridine ligands (∠C1−C3−C4≈176–179°).
In comparison, stirring a THF solution of 0.5 equivalents of Si2C6 and 3 for 24 h at 25 °C resulted in only 20 % conversion to a symmetrical species (by 1H and 19F NMR analysis). Heating the reaction mixture to 45 °C resulted in full conversion after 46 hours (Scheme 3). Unlike complex 8, this new complex remained dissolved in solution and was isolated as dark maroon crystals in 76 % yield after work up. Diagnostic evidence for formation of the product (9) came from 1H NMR analysis, which revealed a 1 : 1 ratio of the DPFN resonances and a singlet at 3 ppm integrating to four protons. X-ray quality crystals were obtained by the slow evaporation of a THF solution of 9 at 25 °C, allowing solid-state structural confirmation of the proposed assignment (Figure 7, bottom).
The solid-state structure of 9 consists of a 1,5-hexadiyne linker connecting two (DPFN)Cu2 units, with a dihedral angle ∠C2−C3−C3′−C2′=180.0(3)°. To the best of our knowledge, no copper-based structures containing this C6H42− fragment have previously been reported. Given the C1−C2 distances (1.209(5) Å, comparable to those observed in 8), complex 9 can be regarded as a more “flexible” version of species 8. Both complexes were characterized by mass spectrometry (Figures S19–S20), but only 8 exhibited an observable, but weak, stretch for the alkyne fragment by IR spectroscopy (ν=1897 cm−1, Figure S24).
The electronic structures of the synthesized complexes were investigated by UV/Vis spectroscopy. Figure 8 displays the absorption spectrum in MeCN of complexes 4, 6–9, and free DPFN from 200 to 800 nm. Absorptions from 200 to 320 nm are attributable to the DPFN ligand, as evidenced by the orange trace in Figure 8. Nonetheless, there is a shoulder from 320 to 420 nm for species 4, 6, 7 and 9. In the case of 8, this absorption is much more evident, with a stronger absorbance value at 370 nm. Indeed, similar bands are observed for the tetracopper complexes described in Figure 1, which were assigned to ligand-to-metal charge transfer (LMCT) bands for [(C≡C)2−→Cu4].6, 8 In those examples, excitation at λ >350 nm resulted in green luminescence, with Stokes shift of the emission bands. However, irradiation at λ=350 nm resulted in no emission for 6 or 8. Additionally, pentacopper species 7, which possesses σ and π bonding between the C22− fragment and the copper atoms, exhibits an absorption spectrum similar to that of complex 6, indicating that participation of the fifth metal and the π electron system does not seem to influence the photophysical properties. Considering these results, and the role that copper(I) species play in the production of OLEDs,29 it is of interest to investigate the electronic factors that govern the luminescence properties of these complexes, and this is a planned future direction.
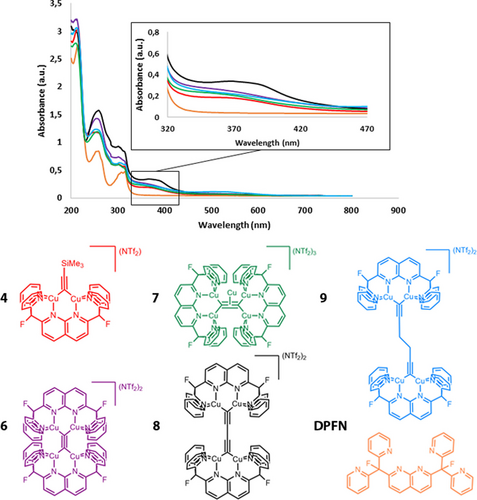
Electronic absorption spectra of complex 4 (red), 6 (purple), 7 (green), 8 (black), 9 (blue) and DPFN (orange), from 200 to 800 nm. Inset: Spectral region from 320 to 470 nm displaying features with weaker absorbance values. Spectra were obtained in the 10−5 M range.
Conclusion
In summary, the dicopper tert-butoxide species 3 is an effective starting point for the synthesis of tetracopper(I) complexes possessing various alkyne-based bridging fragments. The unusual η1:η1 coordination mode of the alkyne moiety with each dicopper unit renders the π electron density of the C≡C linkage available for engagement in subsequent reactivity and modification, which provides an unexplored platform to investigate the participation of multiple metal atoms in the transfer of acetylide and alkyne groups. In addition, acetylide 6 might serve as a molecular model of adsorbed acetylene on copper surfaces,30 or as a tetrametallic structural analogue of Reppe's Cu2C2 catalyst.4, 31 Finally, the binding mode enforced by the DPFN ligand strongly influences the electronic properties of the resulting complexes in comparison with previously reported examples.
Acknowledgments
This work was funded by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division under Contract no. DE- AC02-05CH11231. This project also received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 841154 through a fellowship for P. R. This research used resources of the Advanced Light Source, which is a DOE Office of Science User Facility under contract no. DE-AC02-05CH11231. We acknowledge the National Institutes of Health (NIH) for funding the UC Berkeley CheXray X-ray crystallographic facility under grant no. S10-RR027172, the UC Berkeley College of Chemistry NMR facility (Grants SRR023679A, S10OD024998, and 1S10RR016634-01), and the UC Berkeley Molecular Graphics and Computation Facility (grant no. S10OD023532). We thank Prof. Addison N. Desnoyer, T. Alex Wheeler and Dr. Jaruwan Amtawong for helpful discussions, and Dr Nicholas S. Settineri for crystallographic assistance.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.