Boosting Fe Cationic Vacancies with Graphdiyne to Enhance Exceptional Pseudocapacitive Lithium Intercalation
Jingchi Gao
Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Contribution: Data curation (lead), Formal analysis (equal), Methodology (lead)
Search for more papers by this authorXingru Yan
Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Contribution: Formal analysis (supporting)
Search for more papers by this authorCorresponding Author
Prof. Changshui Huang
Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Contribution: Conceptualization (lead), Methodology (lead), Supervision (lead), Writing - review & editing (lead)
Search for more papers by this authorZhihui Zhang
Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Contribution: Formal analysis (supporting)
Search for more papers by this authorXinlong Fu
Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Contribution: Formal analysis (supporting)
Search for more papers by this authorQian Chang
Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
Contribution: Formal analysis (supporting)
Search for more papers by this authorCorresponding Author
Prof. Feng He
Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
Contribution: Formal analysis (supporting), Methodology (equal)
Search for more papers by this authorMeiping Li
Shandong Provincial Key Laboratory for Science of Material Creation and Energy Conversion, Science Center for Material Creation and Energy Conversion, Institute of Frontier and Interdisciplinary Science, Shandong University, Qingdao, 266237 P. R. China
Contribution: Formal analysis (supporting)
Search for more papers by this authorCorresponding Author
Prof. Yuliang Li
Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorJingchi Gao
Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Contribution: Data curation (lead), Formal analysis (equal), Methodology (lead)
Search for more papers by this authorXingru Yan
Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Contribution: Formal analysis (supporting)
Search for more papers by this authorCorresponding Author
Prof. Changshui Huang
Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Contribution: Conceptualization (lead), Methodology (lead), Supervision (lead), Writing - review & editing (lead)
Search for more papers by this authorZhihui Zhang
Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Contribution: Formal analysis (supporting)
Search for more papers by this authorXinlong Fu
Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Contribution: Formal analysis (supporting)
Search for more papers by this authorQian Chang
Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
Contribution: Formal analysis (supporting)
Search for more papers by this authorCorresponding Author
Prof. Feng He
Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
Contribution: Formal analysis (supporting), Methodology (equal)
Search for more papers by this authorMeiping Li
Shandong Provincial Key Laboratory for Science of Material Creation and Energy Conversion, Science Center for Material Creation and Energy Conversion, Institute of Frontier and Interdisciplinary Science, Shandong University, Qingdao, 266237 P. R. China
Contribution: Formal analysis (supporting)
Search for more papers by this authorCorresponding Author
Prof. Yuliang Li
Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorGraphical Abstract
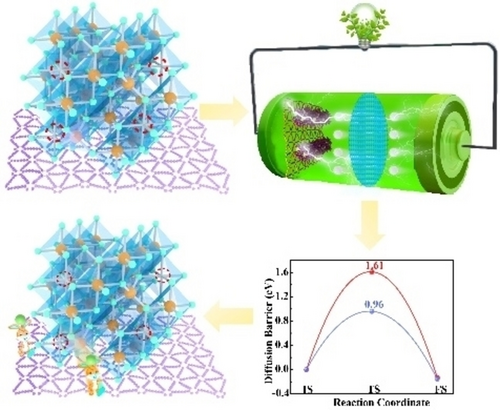
Graphdiyne as anode material in Li-ion batteries can realize effective charge transfer to induce the formation of a high number of Fe vacancies with uniform dispersion. The Fe vacancies modulate charge distribution, serve as active sites and enhance electron-ion transportation, thereby displaying robust pseudocapacitive behavior. The Fe vacancies also reduce the diffusion energy barrier and adsorption energies, leading to a superior battery performance.
Abstract
Modulating the electronic structure of electrode materials at atomic level is the key to controlling electrodes with outstanding rate capability. On the basis of modulating the iron cationic vacancies (IV) and electronic structure of materials, we proposed the method of preparing graphdiyne/ferroferric oxide heterostructure (IV-GDY-FO) as anode materials. The goal is to motivate lithium-ion batteries (LIBs) toward ultra-high capacity, superior cyclic stability, and excellent rate performance. The graphdiyne is used as carriers to disperse Fe3O4 uniformly without agglomeration and induce high valence of Fe with reducing the energy in the system. The presence of Fe vacancy could regulate the charge distribution around vacancies and adjacent atoms, leading to facilitate electronic transportation, enlarge the lithium-ion diffusion, and decrease Li+ diffusion barriers, and thus displaying significant pseudocapacitive process and advantageous lithium-ion storage. The optimized electrode IV-GDY-FO reveals a capacity of 2084.1 mAh g−1 at 0.1 C, superior cycle stability and rate performance with a high specific capacity of 1057.4 mAh g−1 even at 10 C.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202307874-sup-0001-misc_information.pdf2.1 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aY. Xu, M. Zhou, Y. Lei, Adv. Energy Mater. 2016, 6, 1502514;
- 1bN. Kim, S. Chae, J. Ma, M. Ko, J. Cho, Nat. Commun. 2017, 8, 812;
- 1cH. Wu, G. Yu, L. Pan, N. Liu, M. T. McDowell, Z. Bao, Y. Cui, Nat. Commun. 2013, 4, 1943;
- 1dB. W. Zhang, L. Ren, Y. X. Wang, X. Xu, Y. Du, S. X. Dou, Interdiscip. Mater. 2022, 1, 354–372.
- 2
- 2aY. Shen, X. Shen, M. Yang, J. Qian, Y. Cao, H. Yang, Y. Luo, X. Ai, Adv. Funct. Mater. 2021, 31, 2101181;
- 2bX. Li, X. Li, Q. Sun, J. He, Z. Yang, J. Xiao, C. Huang, Acta Phys. Chim. Sin. 2023, 39, 2206029–2206020.
- 3
- 3aF. Wang, Z. Zuo, L. Li, F. He, F. Lu, Y. Li, Adv. Mater. 2019, 31, 1806272;
- 3bS. Xu, C. M. Hessel, H. Ren, R. Yu, Q. Jin, M. Yang, H. Zhao, D. Wang, Energy Environ. Sci. 2014, 7, 632–637;
- 3cC. Yuan, H. B. Wu, Y. Xie, X. W. Lou, Angew. Chem. Int. Ed. 2014, 53, 1488–1504;
- 3dG. Zhang, X. W. Lou, Angew. Chem. Int. Ed. 2014, 53, 9041–9044.
- 4
- 4aY. Shi, J. Zhang, A. M. Bruck, Y. Zhang, J. Li, E. A. Stach, K. J. Takeuchi, A. C. Marschilok, E. S. Takeuchi, G. Yu, Adv. Mater. 2017, 29, 1603922;
- 4bK. Zhang, P. Li, M. Ma, J. H. Park, Adv. Funct. Mater. 2016, 26, 2959–2965;
- 4cG. Zhu, L. Wang, H. Lin, L. Ma, P. Zhao, Y. Hu, T. Chen, R. Chen, Y. Wang, Z. Tie, J. Liu, Z. Jin, Adv. Funct. Mater. 2018, 28, 1800003;
- 4dH. Liu, Q. Li, Z. Yao, L. Li, Y. Li, C. Wolverton, M. C. Hersam, J. Wu, V. P. Dravid, Adv. Mater. 2018, 30, 1704851;
- 4eL. Li, A. Kovalchuk, H. Fei, Z. Peng, Y. Li, N. D. Kim, C. Xiang, Y. Yang, G. Ruan, J. M. Tour, Adv. Energy Mater. 2015, 5, 1500171;
- 4fZ. Peng, Y. Hu, J. Wang, S. Liu, C. Li, Q. Jiang, J. Lu, X. Zeng, P. Peng, F. F. Li, Adv. Energy Mater. 2019, 9, 1802928.
- 5
- 5aD. Han, G. Guo, Y. Yan, T. Li, B. Wang, A. Dong, Energy Storage Mater. 2018, 10, 32–39;
- 5bD. Xia, H. Gao, M. Li, F. Gong, M. Li, Energy Storage Mater. 2021, 35, 169–191;
- 5cJ. Ni, X. Zhu, Y. Yuan, Z. Wang, Y. Li, L. Ma, A. Dai, M. Li, T. Wu, R. Shahbazian-Yassar, J. Lu, L. Li, Nat. Commun. 2020, 11, 1212–1218;
- 5dX. Zhang, D. Wang, X. Qiu, Y. Ma, D. Kong, K. Müllen, X. Li, L. Zhi, Nat. Commun. 2020, 11, 826–3834.
- 6
- 6aX. Gao, Z. Zuo, F. Wang, Q. Chang, H. Pan, L. Li, F. He, Y. Li, Energy Storage Mater. 2022, 45, 110–118;
- 6bB. Wang, Y. Ye, L. Xu, Y. Quan, W. Wei, W. Zhu, H. Li, J. Xia, Adv. Funct. Mater. 2020, 30, 2005834;
- 6cJ. He, L. Luo, Y. Chen, A. Manthiram, Adv. Mater. 2017, 29, 1702707.
- 7
- 7aS. Deng, H. Zhu, G. Wang, M. Luo, S. Shen, C. Ai, L. Yang, S. Lin, Q. Zhang, L. Gu, B. Liu, Y. Zhang, Q. Liu, G. Pan, Q. Xiong, X. Wang, X. Xia, J. Tu, Nat. Commun. 2020, 11, 132–142;
- 7bD. Luo, C. Ma, J. Hou, Z. Zhang, R. Feng, L. Yang, X. Zhang, H. Lu, J. Liu, Y. Li, Y. Zhang, X. Wang, Z. Chen, Adv. Energy Mater. 2022, 12, 2103716;
- 7cT. Tian, L. L. Lu, Y. C. Yin, F. Li, T. W. Zhang, Y. H. Song, Y. H. Tan, H. B. Yao, Adv. Funct. Mater. 2021, 31, 2007419;
- 7dS. Wen, X. Gu, X. Ding, P. Dai, D. Zhang, L. Li, D. Liu, X. Zhao, J. Yang, Adv. Funct. Mater. 2021, 32, 2106751.
- 8
- 8aP. Gao, Z. Chen, Y. Gong, R. Zhang, H. Liu, P. Tang, X. Chen, S. Passerini, J. Liu, Adv. Energy Mater. 2020, 10, 1903780;
- 8bZ. Wu, Y. Zhao, W. Jin, B. Jia, J. Wang, T. Ma, Adv. Funct. Mater. 2020, 31, 2009070;
- 8cY. Fang, Y. Xue, L. Hui, H. Yu, C. Zhang, B. Huang, Y. Li, Adv. Sci. 2021, 2102721.
- 9
- 9aZ. Yang, Y. Song, C. Zhang, J. He, X. Li, X. Wang, N. Wang, Y. Li, C. Huang, Adv. Energy Mater. 2021, 11, 2101197;
- 9bY. Du, Y. Xue, C. Zhang, Y. Liu, Y. Fang, C. Xing, F. He, Y. Li, Adv. Energy Mater. 2021, 11, 2100234.
- 10
- 10aZ. Zhou, Y. Kong, H. Tan, Q. Huang, C. Wang, Z. Pei, H. Wang, Y. Liu, Y. Wang, S. Li, X. Liao, W. Yan, S. Zhao, Adv. Mater. 2022, 34, 2106541;
- 10bF. Wang, J. An, H. Shen, Z. Wang, G. Li, Y. Li, Angew. Chem. Int. Ed. 2022, 62, e202216397.
- 11
- 11aR. Zhang, Y.-C. Zhang, L. Pan, G.-Q. Shen, N. Mahmood, Y.-H. Ma, Y. Shi, W. Jia, L. Wang, X. Zhang, W. Xu, J.-J. Zou, ACS Catal. 2018, 8, 3803–3811;
- 11bJ. Ma, W. Li, B. J. Morgan, J. Światowska, R. Baddour-Hadjean, M. Body, C. Legein, O. J. Borkiewicz, S. Leclerc, H. Groult, F. Lantelme, C. Laberty-Robert, D. Dambournet, Chem. Mater. 2018, 30, 3078–3089.
- 12
- 12aH. Shang, Z. Zuo, L. Yu, F. Wang, F. He, Y. Li, Adv. Mater. 2018, 30, 1801459;
- 12bQ. Yang, Y. Guo, B. Yan, C. Wang, Z. Liu, Z. Huang, Y. Wang, Y. Li, H. Li, L. Song, J. Fan, C. Zhi, Adv. Mater. 2020, 32, 2001755;
- 12cZ. Zuo, F. He, F. Wang, L. Li, Y. Li, Adv. Mater. 2020, 2004379;
- 12dZ. Yang, X. Ren, Y. Song, X. Li, C. Zhang, X. Hu, J. He, J. Li, C. Huang, Energy Environ. Mater. 2023, 6, e12269.
- 13
- 13aY. Liu, Y. Xue, H. Yu, L. Hui, B. Huang, Y. Li, Adv. Funct. Mater. 2021, 31, 2010112;
- 13bL. Qi, Z. Zheng, C. Xing, Z. Wang, X. Luan, Y. Xue, F. He, Y. Li, Adv. Funct. Mater. 2022, 32, 2107179.
- 14
- 14aJ. He, T. Lu, K. Wang, X. Wang, X. Li, X. Shen, J. Gao, W. Si, Z. Yang, C. Huang, Adv. Funct. Mater. 2020, 31, 2005933;
- 14bY. Gao, Y. Xue, T. Liu, Y. Liu, C. Zhang, C. Xing, F. He, Y. Li, Adv. Sci. 2021, 8, 2102777;
- 14cH. Zou, W. Rong, S. Wei, Y. Ji, L. Duan, Proc. Natl. Acad. Sci. USA 2020, 117, 29462–29468.
- 15
- 15aQ. Wei, T. Huang, X. Huang, B. Wang, Y. Jiang, D. Tang, D. L. Peng, B. Dunn, L. Mai, Interdiscip. Mater. 2023, 2, 434–442;
- 15bQ. Wei, X. Chang, D. Butts, R. De Block, K. Lan, J. Li, D. Chao, D.-L. Peng, B. Dunn, Nat. Commun. 2023, 14, 7-15.
- 16D. Yuan, Y. Dou, Y. Tian, D. Adekoya, L. Xu, S. Zhang, Angew. Chem. Int. Ed. 2021, 60, 18830–18837.
- 17
- 17aB. Liu, Q. Zhang, Z. Jin, L. Zhang, L. Li, Z. Gao, C. Wang, H. Xie, Z. Su, Adv. Energy Mater. 2018, 8, 1702347;
- 17bM. Li, H. Du, L. Kuai, K. Huang, Y. Xia, B. Geng, Angew. Chem. Int. Ed. 2017, 56, 12649–12653;
- 17cX. Xiong, L. Yang, G. Liang, Z. Liu, Z. Yang, R. Zhang, C. Wang, R. Che, Adv. Energy Mater. 2022, 12, 2201967;
- 17dZ. Liu, X.-Y. Yu, U. Paik, Adv. Energy Mater. 2016, 6, 1502318.