MET-Activating Ubiquitin Multimers
Graphical Abstract
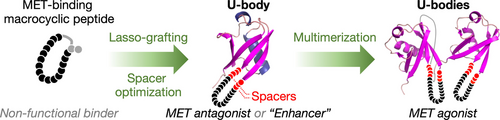
Monomeric small protein ligands, referred to as U-body constructs, were developed from ubiquitin and macrocyclic peptide pharmacophores via genetic implantation (lasso-grafting) and spacer optimization, showing antagonist or “enhancer” activity for a receptor tyrosine kinase MET. Subsequently, multimeric U-body-based proteins, U-bodies, were constructed, exhibiting potent agonist activity for MET.
Abstract
Receptor tyrosine kinases (RTKs) are generally activated through their dimerization and/or oligomerization induced by their cognate ligands, and one such RTK hepatocyte growth factor (HGF) receptor, known as MET, plays an important role in tissue regeneration. Here we show the development of ubiquitin (Ub)-based protein ligand multimers, referred to as U-bodies, which act as surrogate agonists for MET and are derived from MET-binding macrocyclic peptides. Monomeric Ub constructs (U-body) were first generated by genetic implantation of a macrocyclic peptide pharmacophore into a structural loop of Ub (lasso-grafting) and subsequent optimization of its flanking spacer sequences via mRNA display. Such U-body constructs exhibit potent binding affinity to MET, thermal stability, and proteolytic stability. The U-body constructs also partially/fully inhibited or enhanced HGF-induced MET-phosphorylation. Their multimerization to dimeric, tetrameric, and octameric U-bodies linked by an appropriate peptide linker yielded potent MET activation activity and downstream cell proliferation-promoting activity. This work suggests that lasso-grafting of macrocycles to Ub is an effective approach to devising protein-based artificial RTK agonists and it can be useful in the development of a new class of biologics for various therapeutic applications.
Introduction
Receptor tyrosine kinases (RTKs) are a family of single transmembrane receptors which play essential roles in the regulation of most fundamental cellular processes.1 RTKs are generally activated through receptor dimerization and/or oligomerization induced by their cognate ligands, such as hormones or growth factors. Hepatocyte growth factor (HGF) is one such ligand and it activates an RTK MET.2 HGF binds to the extracellular domain of MET to bridge two MET molecules,3 and the resulting MET dimer undergoes autophosphorylation at tyrosine residues within the intracellular kinase domain and the substrate docking site.4 The phosphorylated state of MET then activates the downstream signaling pathways, leading to cellular responses such as cell proliferation, migration, and differentiation.5, 6 Because MET activation is involved in wound repair, HGF has long been regarded as a potential therapeutic agent for regenerative treatments.7 The therapeutic effect of recombinant HGF has been demonstrated not only in preclinical models6 but also in patients with specific diseases.8, 9
Despite the promising potential of HGF, its high cost of production is not necessarily ideal for practical applications. The expression hosts for HGF are restricted to insect or mammalian cells due to the large (≈80 kDa) and complex multidomain structure,10, 11 which results in a high production cost of recombinant HGF.10, 12 Moreover, the intrinsic physical and biological instability of HGF is another issue. To circumvent such problems, there is great interest in developing surrogates for HGF, and so far several artificial MET agonists have been reported, such as engineered HGF splice variants,13, 14 HGF-derived engineered ligand,15 DNA aptamers,16, 17 and peptides.18 We indeed reported the development of potent artificial MET agonists using MET-binding de novo macrocyclic peptides.18 The macrocyclic peptides were identified from the RaPID (random non-standard peptides integrated discovery) system,22 which combines mRNA display and genetic code reprogramming. This method enables us to find nearly optimized sequences of ligands of interest, in this case MET, from the first shot of selection in a few weeks. The potent ligand was then chemically cross-linked to produce a dimer that is a fully functional agonist for MET.
Recently, it has been shown that such RaPID-derived macrocyclic peptides were graftable to protein scaffolds.19-21, 23 The RaPID-derived pharmacophore sequences of the macrocyclic peptides can be genetically implanted into surface-exposed loops of proteins without losing both functionalities of the grafted peptide and the scaffold protein. By this macrocyclic peptide grafting, termed “lasso-grafting (LG)”, the RaPID-derived MET-binding peptides have been exploited to develop designer MET agonists using dimeric and self-assembling proteins as scaffolds.20, 21 A recent example showed that the fragment crystallizable (Fc) region of human immunoglobulin (Ig), which is an intrinsically dimeric protein, was turned into MET agonists without a loss of the binding ability to neonatal Fc receptor.21
In this study, we lasso-grafted the pharmacophore sequence of two distinct MET-binding macrocyclic peptides aMD4 and aMD5 (Figure S1) to ubiquitin (Ub), a robust eukaryotic small protein that is expressed as tandemly conjugated forms,24, 25 to generate Ub-based ligands, referred to as U-body. Unlike the LG to Fc, the initially designed U-body constructs showed substantially weaker affinity to MET compared with the parental peptides. To overcome this stumbling block, we have performed in vitro selection of a U-body library whose flanking spacer sequences were randomized while the pharmacophore sequence was kept intact on the Ub scaffold; and this campaign successfully led to species where the original strong affinity of the macrocycles was fully restored. Taking advantage of the innate feasibility of multimerization of Ub, we generated fully functional MET agonists, referred to as U-bodies, using the affinity-restored MET-binding U-body constructs.
Results and Discussion
First, we investigated the availability of each loop position within Ub for LG using the pharmacophore sequence of aMD4. Six types of aMD4-grafted Ubs were expressed in Escherichia coli, where two consecutive residues within one of the six loop positions were replaced with the pharmacophore sequence of aMD4 flanked by a single Gly as a grafting spacer (Figure S2). Among the six loop positions in Ub, the β1–β2 loop showed the highest tolerance for the pharmacophore insertion to give a high expression yield comparable to that of the wild-type Ub. Based on this observation, the β1–β2 loop was chosen as the grafting position for further studies.
As an initial trial, we prepared a family of U-body in a straightforward design manner (Figure 1a).19-21, 23 The pharmacophore sequence of aMD4 or aMD5 was flanked by “G” or “GG” spacers and it replaced L8-T9 or T9-G10 within the β1–β2 loop (Ub-aMD4_n1–4 and Ub-aMD5_n1–4, referred as naïve U-body, Figure 2a–b). The binding affinity was measured by means of surface plasmon resonance (SPR), which revealed that the naïve U-body constructs showed binding to MET but with substantially weaker affinities than those of the parental macrocyclic peptides (Figure 2a–b and S3); e.g., the dissociation constant (KD) of the Ub-aMD4_n1–4 was >100 nM and that of the Ub-aMD5_n1–4 was >1,200 nM, which were >50-fold and >500-fold higher than that of the respective parental peptides. On the other hand, the naïve U-body constructs showed almost identical circular dichroism spectra to the wild-type Ub (Figure S4), suggesting that they likely maintained the correct folding of Ub. These results indicate that this affinity depletion was not due to the misfolding but was due to the conformational perturbation of the lasso-grafted pharmacophores. Rather, we suspected that the loss of binding activity could be attributed to partial disruption of a structurally active form of the pharmacophore induced by the LG into the Ub site. In consideration of grafting spacer residues playing a critical role in maintaining the active “macrocyclic-like” form of the pharmacophore,26 we envisioned that the loss of binding activity could be fixed by optimizing the flanking spacers at the grafting site.
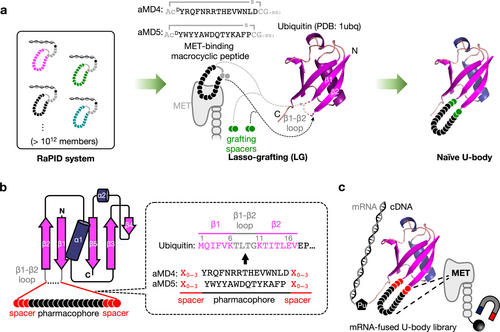
Lasso-grafting of a peptide pharmacophore originating from a RaPID peptide to Ub and its grafting spacer library. (a) Schematic illustration of LG of MET-binding macrocycles to Ub in this study. The parental MET-binding macrocyclic peptides, aMD4 and aMD5, were obtained from the RaPID system in the previous study.18 The pharmacophore sequences (black) were flanked by grafting spacer sequences and genetically implanted into the β1–β2 loop of Ub. The DTyr located at the N-terminus of the pharmacophore sequence was replaced with LTyr according to the previous studies.19-21 (b) Construction of the Ub-aMD4 and Ub-aMD5 library. The pharmacophore sequences were flanked by 0 to 3 randomized grafting spacer residues (represented as “X′′) and they replaced 0 to 4 consecutive residues within the β1–β2 loop (T7 to G10). The complete sequences of the mRNA libraries are described in Figure S5a–b. (c) Schematic illustration of the mRNA display strategy for the discovery of MET-binding U-body.
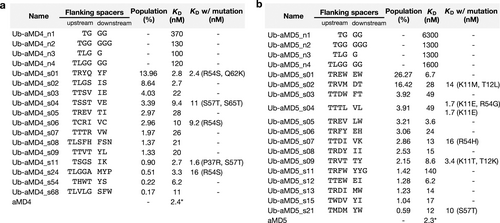
Binding affinity of each U-body construct measured by SPR. (a) U-body with the pharmacophore sequence of aMD4 (Ub-aMD4). (b) U-body with the pharmacophore sequence of aMD5 (Ub-aMD5). The flanking spacers represent the sequence between K6 and the pharmacophore region (upstream), and that between the pharmacophore region and K11 (downstream). The population (%) indicates the sum of the proportion of U-body sequences bearing the flanking spacers in the recovered library after the selection. * Values for aMD4 and aMD5 are taken from Ito et al. (2015).18
To perform the optimization of the flanking spacer sequences of the pharmacophore, we adopted mRNA display to reselect new flanking spacer sequences on the U-body scaffold with superior binding abilities. Two mRNA libraries encoding aMD4-grafted U-body (Ub-aMD4) or aMD5-grafted U-body (Ub-aMD5) with a variety of flanking spacer sequences were constructed, where 0 to 4 consecutive residues within the β1–β2 loop were replaced with the pharmacophore sequence flanked by randomized 0–3 spacer residues (Figure 1b, S5a–b). The libraries were applied for the standard mRNA display selection, where the ectodomain of human MET (Fc fusion) was used as the target molecule as in the previous in vitro selection that identified aMD4 and aMD5 (Figure 1c, S6).18 Note that the theoretical diversity of the libraries was 9.9×108, which could be fully covered by the practical number of variants (>1011) translatable by the in vitro translation cocktail. Therefore, all possible combinations were exhaustedly explored by the selection.
After six iterative rounds of the selection, the entire region of each cDNA library was sequenced by a next-generation sequencer (Figure S6). The sequence analysis revealed that there was a strong preference for the replaced residues within the β1–β2 loop and the number of spacer residues. In the Ub-aMD4 library, TXXX-aMD4-XX (the Ub-aMD4 sequences whose upstream flanking spacer sequence of the pharmacophore matches TXXX and whose downstream flanking spacer sequence matches XX, where X denotes a random spacer residue) and TLXX-aMD4-XX rapidly increased their population as the selection proceeded, and they reached 80 % and 10 % of the library after the sixth round, while they comprised less than 0.1 % of the initial library (Figure S7a–b). TLXXX-aMD4-XXX also remained after the sixth round and comprised 8 % of the library. In contrast, XXX-aMD4-XXX completely disappeared, although it comprised the largest fraction (37 %) in the initial library. Similar to the Ub-aMD4 library, the Ub-aMD5 library was mainly dominated by TXXX-aMD5-XX (93 %) after the selection (Figure S7c–d).
We also analyzed the mutations accumulated in the constant regions during the in vitro selection (Figure S8 and S9). In both libraries, a large part of the sequences (84 % of the Ub-aMD4 library and 59 % of the Ub-aMD5 library) contained at least one mutation in the scaffold region after the selection (Figure S10). Most of the mutated residues were structurally distant from the β1–β2 loop and were less likely to contribute to the affinity to MET. Mutations in K11 and T12 (the residues next to the β1–β2 loop) have appeared in some of the most abundant sequences originating from the Ub-aMD5 library, such as Ub-aMD5_s02-K11M T12L A46G G57S L67V, Ub-aMD5_s04-K11E R54G, and Ub-aMD5_s09-K11T T12K R54S.
From the enriched Ub-aMD4 and Ub-aMD5 libraries, 13 and 14 U-body constructs were chosen for affinity measurement. The selected U-body constructs were expressed in the absence of the scaffold mutation(s). SPR experiments demonstrated that all selected U-body constructs exhibited MET binding affinities in a nanomolar range (Figure 2a–b, S11). Remarkably, Ub-aMD4_s02 showed the highest binding affinity among the Ub-aMD4 constructs, with a KD of 2.7 nM, which is comparable to aMD4 and >37-fold higher affinity than the naïve Ub-aMD4 constructs (KD >100 nM). Similarly, Ub-aMD5_05 exhibited an affinity of 3.6 nM, which is >350-fold higher affinity than the naïve Ub-aMD5 constructs (KD >1300 nM). These results indicate that the optimization of the flanking spacer sequences successfully worked to restore the binding affinity to the pharmacophores. Interestingly, Ub-aMD4_s06 having two Cys residues in the spacer region would render a constrained structure on the pharmacophore sequence by forming a disulfide bond, but it possesses 10 nM KD that is a higher value than those with non-covalent spacers, such as Ub-aMD4_s02. This suggests that the selected non-covalent spacer sequences provide a sufficient constraint capable of bringing both termini of the pharmacophore close together and displaying the pharmacophore as a Ub's loop in a suitable conformation for binding.
For the U-body sequences that contained a mutation(s) within the constant regions of the sequenced clone, we also attempted to prepare constructs with the scaffold mutation(s) to evaluate their effect on the affinity (Figure 2a–b). SPR experiments revealed that Ub-aMD5 constructs with the scaffold mutation in K11 or K11 and T12 exhibit higher affinity than without the scaffold mutations, while the other mutations did not significantly affect the affinity. In particular, Ub-aMD5_s04-K11E R54G exhibited a 29-fold lower KD (1.7 nM) than Ub-aMD5_s04 without the scaffold mutation. As R54 is structurally distant from the β1–β2 loop, we also prepared Ub-aMD5_s04-K11E for evaluation. Ub-aMD5_s04-K11E exhibited the same affinity as Ub-aMD5_s04-K11E R54G (KD=1.7 nM), while the removal of R54G improved the thermal stability (Tm=48 °C to 60 °C, Figure S12). Considering the results of the above evaluations and expression behaviors, we chose the two Ub-aMD4 constructs (Ub-aMD4_s02 and Ub-aMD4_s24) and the two Ub-aMD5 constructs (Ub-aMD5_s01 and Ub-aMD5_s04-K11E) for further assays.
Next, we evaluated the binding ability of the above U-body constructs to MET on live cell surfaces. A S20 C mutant of each construct was expressed and conjugated with fluorescein-5-maleimide to yield the corresponding fluorescent-labeled U-body (U-body-F). MET knock-out (KO) and MET overexpressing (OE) human lung adenocarcinoma PC-9 cells were incubated with the respective U-body-F and its binding was evaluated by flow cytometry. All constructs of the U-body-F showed a MET-expression-dependent signal increase while the parental Ub-F did not show such a signal increase (Figure 3a). The binding of the U-body-F constructs was also evaluated by fluorescent microscopy. The MET-OE and MET-KO PC-9 cells were mixed and incubated with the respective U-body-F constructs. The fluorescence images show that the U-body-F constructs were selectively internalized into MET-OE cells (Figure S13). To further evaluate their binding ability, the U-body-F constructs were intravenously administered to hHGF knock-in SCID mice bearing MET-KO and MET-OE PC-9 cells. Indeed, the fluorescent signal from the U-body-F constructs was specifically observed in only the MET-OE cells in SCID mice (Figure 3b). These results show that the U-body constructs can bind to MET on the live cell surface not only under in vitro conditions but also in vivo environments.
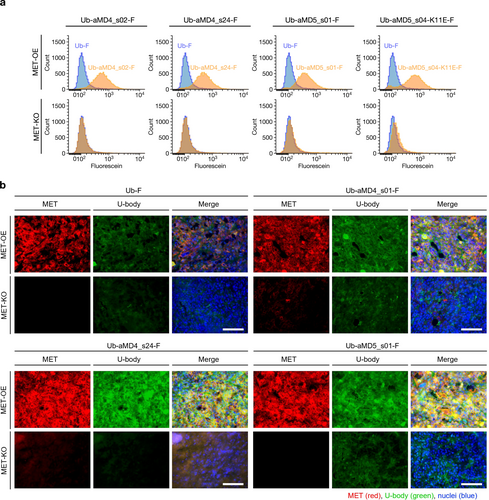
Binding ability of U-body to cellular MET. (a) Cell-based binding assay of the U-body-F constructs. MET-OE or MET-KO PC-9 cells were incubated with the U-body-F constructs and analyzed by flow cytometry. (b) In vivo binding assay of the U-body constructs. The U-body-F constructs were administered to hHGF knock-in SCID mice bearing MET-OE and -KO PC-9 (i.v., 10 μg/head). Each image was acquired after the staining of U-body (anti-His-tag mouse mAb and Alexa 488-labeled anti-mouse goat antibody), MET (anti-MET rabbit antibody and Alexa 594-labeled anti-rabbit goat antibody) and nuclei (DAPI). Scale bars, 100 μm.
In vivo or cellular systems, HGF dimerizes MET to induce trans-phosphorylation at the Y1234/1235 residues in the activation loop, transducing the biological signals to downstream events (Figure 4a). The aMD4 and aMD5 peptides are potent binders of MET but they could not antagonize the MET activation by HGF (Figure 4b and c, blue line). Based on the potent binding ability of U-body constructs to cellular MET, we wondered if the additional steric effect of the Ub body part could grant an inhibitory function to the U-body construct. EHMES-1 cells stably expressing MET were stimulated by 1 nM HGF in the presence of various concentrations of U-body, and ELISA (enzyme-linked immunosorbent assay) method was used to detect the phosphorylation level of the Y1234/1235 residues. Interestingly, Ub-aMD4_s02 showed a partial inhibitory activity (IC50=67 nM) where phosphorylation was reduced to a 60 % level and then became a plateau (Figure 4b, orange line). In contrast, Ub-aMD4_s24 exhibited an enhancing ability of phosphorylation of HGF by 1.5-fold (Figure 4b, green line), whereas it alone (no HGF) did not induce the MET phosphorylation (Figure S14). This observation indicates that the observed MET enhancement is a cooperative effect with HGF and suggests that Ub-aMD4_s24 stabilizes the active MET-HGF complex. This is presumably because its long spacers place the Ub body part in close proximity to HGF, causing some interaction between them. It should be noted that the binding site of aMD4 was mapped to the lower face of the Sema domain of MET,21 which is close to the binding sites of the K1 and the K3 domains of HGF. On the other hand, both Ub-aMD5_s01 and Ub-aMD5_s04-K11E showed complete inhibitory activity against the MET activation by HGF (Figure 4c, orange line with IC50=240 nM and green line with IC50=82 nM, respectively), indicating that its peptide binding site is effective to generate steric crush by the Ub body part against HGF.
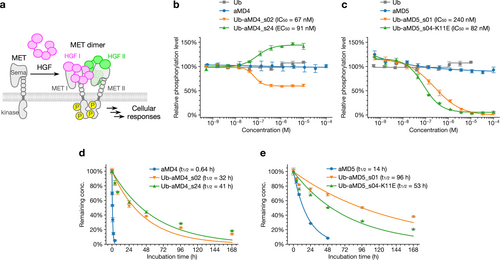
Biochemical properties of U-body. (a) Activation of MET by HGF. MET and HGF form the asymmetric 2 : 2 complex. The dimeric MET undergoes autophosphorylation and activates downstream signaling pathways, leading to cellular responses. (b–c) Antagonist activity assay of Ub, the parental peptides, and the U-body constructs. EHMES-1 cells were stimulated with 1 nM HGF in the presence of each molecule for 10 min. The MET phosphorylation levels were quantified by cell-based ELISA with anti-phospho-MET (Tyr1234/1235) antibody. The MET phosphorylation level induced by 1 nM HGF was defined as 100 %. IC50 and EC50 values are also indicated. Error bars, s.d. (n=3). (d–e) Serum stability assay of the parental peptides and the U-body constructs. The time courses represent the percentage of the remaining active concentration after incubation in human serum at 37 °C. At the designated time points, the remaining active concentration of the macrocyclic peptides or the U-body constructs was measured by SPR. The mean at 0 h was defined as 100 %. Half-lives, t1/2, are also indicated. Error bars, s.d. (n=3).
Next, we evaluated the stability of the U-body constructs in human serum and compared them with that of aMD4 and aMD5. Each U-body construct or macrocyclic peptide was incubated in human serum at 37 °C. At each time point, their remaining active concentrations were quantified using SPR (Figure 4d–e). In contrast to the short-medium half-lives of aMD4 and aMD5 (t1/2=0.64 h and 14 h), all tested U-body constructs showed significantly prolonged half-lives in serum (t1/2=32 h, 41 h, 96 h, and 53 h for Ub-aMD4_s02, Ub-aMD4_s24, Ub-aMD5_s01, and Ub-aMD5_s04-K11E, respectively). This result indicates that LG of the peptide pharmacophores to the Ub scaffold is able to grant higher serum stability than the parental macrocycles.
As Ub can be expressed as repeated sequence forms in nature,24 we reasoned that multimerization would also be amenable to U-body, and such multimers of U-body, referred to as U-bodies, would work as MET agonists capable of dimerizing/multimerizing MET for downstream signal inductions (Figure 5a). Because the linker connecting each binding moiety potentially affects MET agonist activity similar to peptide dimers of aMD4 and aMD5,16 we adopted three types of well-established peptide linkers in various lengths [(GGGGS)1–5, non-charged, flexible; (EAAAK)1–5, charged, rigid; and A(PA)3,5,7,9,11, non-charged, rigid] accompanied with a short adapter sequence (TS) to connect the U-body units. The dimeric constructs of Ub-aMD4_s02, Ub-aMD4_s24, and Ub-aMD5_s01 (referred to as diUb-aMD4_s02, diUb-aMD4_s24, and diUb-aMD5_s01, respectively), were obtained in soluble forms, but that of Ub-aMD5_s04-K11E became an inclusion body in E. coli cells (Table S3); therefore, we focus on the former three dimeric U-bodies for further studies. The respective dimeric U-bodies at 20 nM were incubated with EHMES-1 cells, and their phosphorylation level of MET was compared with that induced by 1 nM HGF. Remarkably, all the dimeric U-bodies induced substantial phosphorylation regardless of the type or length of the linker (Figure S15a–c). In diUb-aMD4_s24, higher phosphorylation levels were observed as the linker became longer (Figure S15b), but such a trend was not observed in diUb-aMD4_s02 and diUb-aMD5_s01 (Figure S15a, c). We have previously shown that the peptide aMD5 dimer exhibited a higher agonist activity with a longer linker (≈56 Å) than a short linker (≈13 and ≈30 Å), while the peptide aMD4 dimer showed a similar level of agonist activity regardless of the length of linker.16 The observed activity of diUb-aMD5_s01 (Figure S15c) indicates that the sum of the length of the shortest peptide linker and the adapter sequence (7 amino acids, ≈26 Å) was sufficiently long enough for the induction of MET activation. It is likely that the Ub scaffold itself could also act as a sort of linker for the pharmacophores (the distance between the C-terminus and the β1–β2 loop gives ≈20 Å, and that between the N-terminus and the β1–β2 loop does ≈24 Å).

Biochemical properties of multimeric U-body constructs, U-bodies. (a) Activation of MET by U-bodies. A peptide linker with the adapter sequence (TS) links each U-body unit. U-bodies bridges MET molecules to induce their autophosphorylation. (b–d) MET phosphorylation level after stimulation with (b) diUb-aMD4 constructs, (c) diUb-aMD5 constructs, and (d) multimeric constructs of Ub-aMD4_s24-(EAAAK)5. EHMES-1 cells were stimulated by the respective U-bodies constructs and the phosphorylation level of MET was quantified by a cell-based ELISA. Error bars, s.d. (n=3). (e–f) Cell proliferation assay. HUVEC were cultured in the presence of HGF or a multimeric construct of Ub-aMD4_s24-(EAAAK)5 for four days. The medium was replaced every other day. The cell proliferation in each condition was evaluated by MTS assay. Error bars, s.d. (n=4). *** P<0.001.
We next evaluated the agonist activity of the dimeric U-bodies connected by the (EAAAK)5 linker in a concentration-dependent manner. Similar to the dimers of the parental macrocyclic peptides (aMD4-PEG3, aMD4-PEG11, and aMD5-PEG11; Figure S1c-d, S16a–b), the diUb-aMD4_s02, diUb-aMD4_s24, and diUb-aMD5_s01 constructs with (EAAAK)5 linker [diUb-aMD4_s02-(EAAAK)5, diUb-aMD4_s24-(EAAAK)5, and diUb-aMD5_s01-(EAAAK)5] induced various dose-responses reaching comparable or even higher maximum phosphorylation levels than HGF at the appropriate concentration range (11–33 nM in diUb-aMD4_s02-(EAAAK)5, diUb-aMD4_s24-(EAAAK)5 and 111–333 nM in diUb-aMD5_s01-(EAAAK)5) (Figure 5b–c). This indicates that the dimeric U-bodies are fully functional or even enhanced agonists for MET. In contrast, the naïve dimeric U-bodies (derived from Ub-aMD4_n4 and Ub-aMD5_n4) induced virtually no phosphorylation to MET, indicating that the agonist activity of U-bodies largely relies on the strong affinity of the U-body unit to MET. We also investigated the effect of the linker length on the concentration dependency of the agonist activity of the series of diUb-aMD4_s02, diUb-aMD4_s24, and diUb-aMD5_s01 with the (EAAAK)1–5 linkers. In the case of diUb-aMD4_s02-(EAAAK)1–5 and diUb-aMD5_s01-(EAAAK)1–5, the activation profiles were almost identical regardless of the linker lengths (Figure S16c, e), whereas diUb-aMD4_s24 with the longest (EAAAK)5 linker induced the highest phosphorylation level than shorter ones (Figure S16d).
Encouraged by the successful transformation of monomeric U-body into MET agonist molecules by dimerization, we wondered if the agonist activity could be further enhanced by the multimerization of U-body units to increase the valency. Since Ub-aMD4_s24 did not inhibit MET activation by HGF and diUb-aMD4_s24-(EAAAK)5 showed the best expression behavior, we chose this construct for investigating the multimerization effect. Tetrameric, hexameric, and octameric U-bodies of Ub-aMD4_s24-(EAAAK)5 (tetUb-aMD4_s24-(EAAAK)5, hexUb-aMD4_s24-(EAAAK)5, and octUb-aMD4_s24-(EAAAK)5) were prepared and their activity was evaluated by the MET phosphorylation assay. The ELISA showed that the multi-Ub-aMD4_s24-(EAAAK)5 constructs induced the MET phosphorylation at lower concentrations as the valency increased, indicating that each U-body unit is correctly folded and able to bind to MET in the multimers to participate in their cooperativity and/or avidity (Figure 5d). Remarkably, hexUb-aMD4_s24-(EAAAK)5, and octUb-aMD4_s24-(EAAAK)5 induced similar phosphorylation levels to HGF around 1.1 nM (at 10−9 M in Figure 5d), where HGF shows the maximum activity.
To confirm that the strong phosphorylation activity of the multi-U-bodies constructs elicits the subsequent cellular response of the MET signaling pathway, we performed a cell proliferation assay using the multi-Ub-aMD4_s24-(EAAAK)5 constructs. Human umbilical vein endothelial cells (HUVEC) were cultured in the presence of HGF or respective multimeric U-bodies constructs. Note that since HUVEC were able to self-proliferate due to the production of their own HGF, this was set as the background at 100 % as a vehicle control and then further enhancement of the proliferation was monitored (Figure 5e). Under such experimental conditions, HGF induced cell proliferation on HUVEC and the cell proliferation level reached a 1.6-fold higher level at 1 nM concentration compared to the vehicle control. 1 nM concentration of the multimeric U-bodies constructs were able to promote cell proliferation in similar levels to HGF. We further evaluated the concentration dependency of the cell proliferation activity of diUb-aMD4_s24-(EAAAK)5 and tetUb-aMD4_s24-(EAAAK)5. Notably, the agonist effect of HGF rapidly declines at 20 nM concentration via so-called bell-shaped behavior, where HGF starts forming inactive ligand:receptor 2 : 1 complex rather than active 2 : 2 complex.18 On the other hand, the U-bodies retain the cell proliferation effect at higher concentrations, i.e., their declination of agonist effect is slower than that of HGF, giving a wider range of concentrations for the enhancement of proliferation (Figure 5f).
Conclusion
Ub consists of 76 residues with about 8.6 kDa, and this robust small protein has been utilized as a scaffold for de novo non-Ig protein ligands.25, 27, 28 Its surface residues have been subjected to randomization for the construction of libraries, which have been applied to screening against proteins that are known to weakly interact with Ub, such as ubiquitin ligases and deubiquitinases, to obtain Ub-based inhibitors.29-32 However, targeting proteins other than such Ub-interacting proteins using such surface-randomized libraries has appeared to be more challenging, presumably because the shape and area of the potential interaction interface are restricted by the folding of the small globular scaffold. The binding affinity of reported Ub variants with seven to eight mutations was generally weak as protein ligands (KD >100 nM).33, 34 Although sub- to low nanomolar affinities could be achieved by increasing the number of mutations to expand the potential interface area, such successful examples are still limited.35, 36 In such cases, Ub variants were dimeric and contained 15 mutations across the two Ub moieties,35 or monomeric but as many as 22–24 residues out of the 76 residues of the scaffold, which represents 29–32 % of the total residues, were mutated.36
In contrast, the LG strategy reported in this study utilizes fairly short pharmacophore sequences (10–15 residues) derived from de novo macrocyclic peptides generated by the RaPID system and allows the specific interaction with the target protein. Therefore, the interaction interface should only rely on the grafted pharmacophore without extensive mutation on Ub itself. Although LG of pharmacophore sequences into loops of larger and more dynamic proteins, e.g. fragment crystallized (Fc) regions (about 50 K Da), resulted in a small loss of binding affinity with arbitrary short spacers,19 LG into a loop of Ub with arbitrary spacers (referred to as “naïve grafting”) suffered from a large loss of activity. This could be credited to more compact and less dynamic properties of the Ub scaffold, which may hinder the grafted pharmacophore from adopting the optimal conformation for the binding. However, their exquisite affinity can be fully exploited by optimizing the flanking short spacer sequences from the exhaustive, diverse library (109) by mRNA display. Importantly, such lasso-grafted pharmacophores generally exhibited low nanomolar KD values on the Ub scaffold with no mutation or only a few mutations occurring near the grafting site.
Notably, minimal or no change of the Ub scaffold has enabled it to retain the parental Ub-like high thermal stability. Interestingly, three out of four monomeric lasso-grafted Ub molecules, referred to as U-body, exhibited inhibitory activity even though the parental peptides (aMD4 and aMD5) did not show such an inhibitory activity at all. Such an effect of the U-body constructs can be attributed to a steric impact between the body of the Ub scaffold with the HGF ligand. This observation in turn suggests the possibility that LG of inhibitory macrocyclic peptides generated by the RaPID system to the Ub scaffold may allow the enhancement of their inhibitory activity.
The observed potent binding ability of U-body constructs against MET allowed us to generate their multimers, referred to as U-bodies. The dimeric U-bodies exhibit agonist activity for MET. Although we previously reported a lasso-grafted Fc with the same MET agonist activity,21 the U-bodies constructs are functionally and structurally different. The lack of ability to bind to Fc receptors eliminates the possibility of unnecessary interference with the immune system functions. In addition, the absence of sugar modification and disulfide bonds allows for easy production using the E. coli expression system and the latter point also ensures their activity even under reducing environments. Importantly, the valency of U-bodies is programmable. Indeed, we were able to generate dimeric, tetrameric, hexameric, and octameric U-bodies with various peptide linkers of choice, and these multimeric U-bodies exhibited exquisite agonist activity even superior to the naturally occurring HGF ligand. Since the construction of U-bodies is modular, we envision that different functional units of U-body can be programmable, yielding bifunctional and even multifunctional U-bodies. Although Ub itself exists in healthy human serum37 and has been proven to be safe for short-term administration to an animal model,38 the immunogenicity, safety, and pharmacokinetics of U-bodies need to be further investigated in the future for practical applications. Knowing such properties will accelerate the development of a new class of biologics (“neobiologics”) based on U-bodies for various therapeutic applications.
In summary, we have demonstrated that LG is amenable to the small protein scaffold Ub without the loss of affinity of the parental macrocyclic peptide, and the resulting U-body constructs could be exploited to develop MET agonist molecules by making their multimeric U-bodies. As the strategy presented here is not confined to aMD4 and aMD5, this study provides a versatile way to develop monomeric U-body constructs and multimeric U-bodies targeting a variety of proteins, contributing to expand the toolbox for devising artificial RTK agonists as well as antagonists for other proteins.
Supporting Information
The authors have cited additional references within the Supporting Information.39, 40
Acknowledgments
This work was supported by Japan Society for the Promotion of Science KAKENHI (JP21J11472 to N. K.; JP21K15482 to H.Sato; JP21K18250 to K. M; JP20H05618 to H. Suga); grants from Japan Agency for Medical Research and Development (JP21cm0106201 to N. T and K. M.; JP23ama221402 to H. Sato, K. M. and H. Suga); Extramural Collaborative Research Grant of Cancer Research Institute, Kanazawa University to N. T. and K. M.; and Grants-in-Aid from The Noguchi Institute to N. T.
Conflict of interest
N. K. and H. Suga. are listed as co-inventors on a provisional patent application related to this work. H. Suga. is a co-founder and shareholder of MiraBiologics Inc.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.