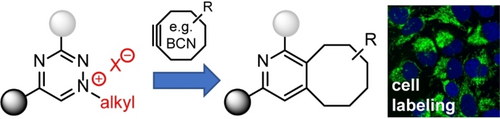
Triazinium Ligation: Bioorthogonal Reaction of N1-Alkyl 1,2,4-Triazinium Salts**
A previous version of this manuscript has been deposited on a preprint server (https://doi.org/10.26434/chemrxiv-2023-75pjq).
Graphical Abstract
We describe N1-tert-butylated 1,2,4-triazinium salts as new bioorthogonal reagents. These heterodienes are stable under biological conditions and react selectively and at high rates with strained alkynes such as BCN, allowing efficient modification of biomolecules. The unique ionic character makes the compounds more water soluble and cell permeable. These features enable efficient labeling of intracellular targets in living cells.
Abstract
The development of reagents that can selectively react in complex biological media is an important challenge. Here we show that N1-alkylation of 1,2,4-triazines yields the corresponding triazinium salts, which are three orders of magnitude more reactive in reactions with strained alkynes than the parent 1,2,4-triazines. This powerful bioorthogonal ligation enables efficient modification of peptides and proteins. The positively charged N1-alkyl triazinium salts exhibit favorable cell permeability, which makes them superior for intracellular fluorescent labeling applications when compared to analogous 1,2,4,5-tetrazines. Due to their high reactivity, stability, synthetic accessibility and improved water solubility, the new ionic heterodienes represent a valuable addition to the repertoire of existing modern bioorthogonal reagents.
Introduction
Chemical reactions compatible with biological systems have numerous applications. They are employed in the modification of biopolymers,1 the visualization of small molecules and biomolecules inside cells2 and also in biomedicine.3 To proceed efficiently and selectively, the reagents involved in these reactions must be sufficiently reactive and stable in biological media. In recent decades, numerous biocompatible reagents and reactions have been developed.4 Among these, various strain-driven cycloadditions have become popular for their ability to proceed cleanly and selectively (even in a crowded biological environment) without the need for an additional catalyst. For example, the strain-promoted azide-alkyne cycloaddition (SPAAC)5 and its variations employing other dipoles6 have found application in a wide range of fields. Another popular cycloaddition is the inverse electron-demand Diels–Alder reaction (IEDDA) of 1,2,4,5-tetrazines with strained dienophiles.7 Although a number of strained olefins have been developed as reaction partners in IEDDA,8 there are far fewer examples of dienes that can be used in this reaction. Besides 1,2,4,5-tetrazines, derivatives of 1,2,4-triazines have been developed as more stable alternatives.9 However, the improved stability of triazines is associated with reduced reaction rates.
Due to the inverse electron demand characteristic of IEDDA, the attachment of electron-withdrawing substituents to the heterocyclic core enhances the reactivity of the dienes in the cycloaddition. This strategy has been successfully utilized to improve the reactivity of both, 1,2,4-triazines9a, 10 and 1,2,4,5-tetrazines.11 However, especially for tetrazines, these modifications also increase the susceptibility to nucleophilic attack at the electron-deficient positions 3- and 6-, which can lead to their degradation in biological media.12 Therefore, there is a growing demand for new strategies that increase the reactivity of bioorthogonal reagents without reducing their stability.
Recently, a distortion caused by a repulsive N−O interaction of vinyl ether-substituted tetrazines was described as a strategy to increase their reactivity without impeding their stability.13 Another elegant solution for generating highly reactive tetrazines is based on light-triggered photoactivation of stable dihydrotetrazine precursors.14 More recently, dihydrotetrazines modified with photocaging groups as precursors to 1,2,4,5-tetrazines have been proposed (Figure 1).15 In spite of these advances, the development of highly reactive and at the same time stable bioorthogonal reagents remains challenging. Moreover, similar strategies for 1,2,4-triazines have yet to emerge.
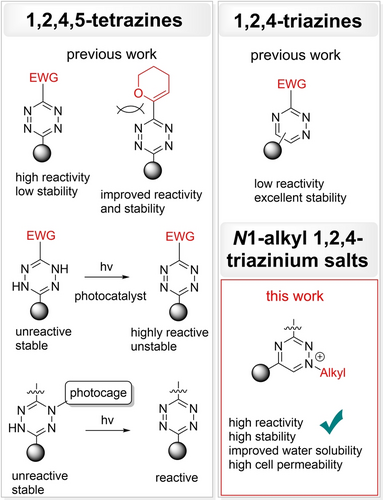
Previous and new strategies for improving the reactivity and stability of heterodienes in bioorthogonal reactions.
Two other important parameters, especially for intracellular application of bioorthogonal reactions, are the solubility and cell permeability of the reagents. Water solubility is typically enhanced by the attachment of polyethylene glycol (PEG) linkers of various length to molecules. However, this increases the molecular weight of the compounds, which can influence their (bio)distribution, impede their ability to cross cell membranes and even cause an immune reaction.16 An alternative strategy involves the modification of molecules by charged moieties, such as sulfo groups. Indeed, a number of commercial tetrazine derivatives contain sulfo groups, e.g. sulfonated dyes. However, these effectively render compounds cell-impermeable, which limits their use mainly to fixed and permeabilized cells. By contrast, the introduction of positive charge(s), which can significantly enhance the water solubility and cell permeability17 of molecules, is much less common. Previously, we demonstrated that the attachment of a charged pyridinium moiety to 1,2,4-triazines yields a notable group of water-soluble, cell-permeable compounds with enhanced applicability in bioconjugations.10 More recently, we extended this approach to positively-charged mitochondriotropic tetrazines, which can activate trans-cyclooctene-caged prodrugs in an organelle-specific manner.18 These examples indicate that positively charged groups can endow molecules with advantageous properties. Yet, in the context of bioorthogonal reagents, these effects remain largely unexplored.
In this work, we show that selective alkylation of 1,2,4-triazines at nitrogen N1 yields the corresponding N1-alkyl triazinium salts (Trz+s), which are not only stable under biological conditions, but also highly reactive in reaction with strained alkynes. In addition, the compounds have improved water solubility while displaying high cell-permeability (Figure 1). A head-to-head comparison of the triazinium salts with the popular 1,2,4,5-tetrazines bearing analogous substituents shows that the new reagents outperform the latter compounds in intracellular labeling experiments. As we demonstrate in several examples, these features make Trz+s expedient bioorthogonal reagents in a wide range of applications, from selective peptide and protein labeling to cellular imaging.
Results and Discussion
We and others have previously used triazinium salts as precursors to the corresponding triazinium ylides, which are formed in situ under basic conditions. These dipoles react in 1,3-dipolar cycloadditions with electron poor dipolarophiles to yield pyrrolotriazines.19 Alternatively, an intramolecular cycloaddition of triazinium salts leading to substituted pyridines has been described.20
We sought to exploit triazinium salts as chemoselective reaction partners in bioorthogonal cycloadditions with strained dienophiles. To this end, we carried out a reaction of N1-methyl-3,5-diphenyl triazinium (Trz+1) with endo-bicyclo[6.1.0]non-4-yn-9-ylmethanol (endo-BCN) (Figure 2A). Upon mixing the reagents, we observed a clean conversion to the corresponding pyridine cycloadduct 1 within a couple of minutes (Figure 2B). Compared with the non-alkylated 3,5-diphenyl-1,2,4-triazine (Trz1), the reaction of Trz+1 with endo-BCN was three orders of magnitude faster (k2=0.021 M−1 s−1 and 20 M−1 s−1, respectively). This result shows that alkylation of the triazine heterocycle at nitrogen N1 induces a dramatic increase in reactivity. Notably, alkylation of 3,6-diphenyl-1,2,4,5-tetrazine (diPhTz) under the same conditions was unsuccessful, resulting only in the isolation of the corresponding reduced dihydrotetrazine. By contrast, alkylation of 3,6-diphenyl-1,2,4-triazine was successful, despite the ensuing click ligation with endo-BCN generating a complex mixture of products (Supporting Information, SI).
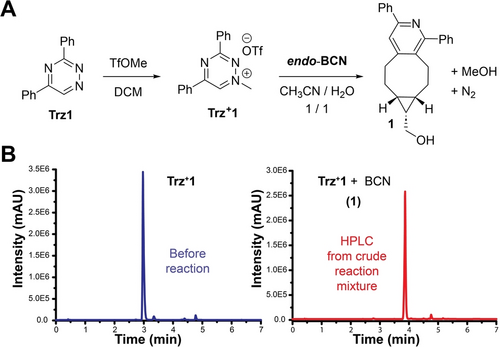
(A) Methylation of 3,5-diphenyl-1,2,4-triazine produces N1-methyl-1,2,4-triazinium salt that efficiently reacts with strained endo-BCN alkyne. (B) HPLC chromatograms show clean conversion of Trz+1 in reaction with endo-BCN giving rise to the corresponding cycloaddition product.
To understand the basis for the increased reactivity of 3,5-disubstituted N1-alkyl triazinium salts, we performed a computational study at the density-functional-theory (DFT) level. We found transition-state (TS) structures for the IEDDA reaction of endo-BCN with Trz1 and with Trz+1. The relative free energy of the TS with the triazinium derivative was 10 kcal mol−1 lower than that of the TS with the triazine starting compound (Figure 3A), which confirms strong preference of endo-BCN to react with the positively charged heterodiene.
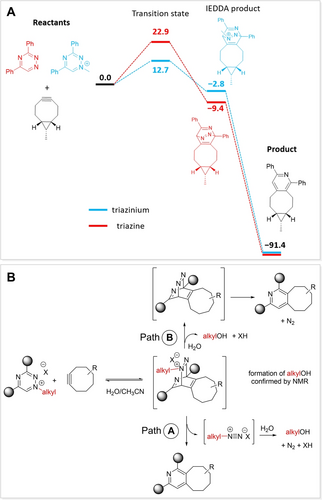
(A) The calculated (B3LYP/6-31+G(2d,p)/CPCM) relative free energies (kcal mol−1) of the reactants, transition-state structures and products of the IEDDA reaction of Trz1 and Trz+1 with endo-BCN (the hydroxymethyl group was substituted with a methyl group for simplification). (B) Proposed reaction mechanism of the triazinium ligation.
The subsequent reaction steps after formation of the cycloaddition product involve either denitrogenation of the triazine intermediate or the departure of the alkyl diazonium species from the triazinium intermediate followed by hydrolysis to give an alcohol and a nitrogen molecule (Path A in Figure 3B). Alternatively, we speculate that hydrolysis may precede the denitrogenation step (Path B in Figure 3B). While evolution of nitrogen gas (bubbling) during the reaction was observed experimentally, presence of the hydrolysis product (an alcohol) was confirmed by NMR spectroscopy (alkyl=ethyl, Figure S2 in the SI). Computational analysis of the reactions following the formation of the IEDDA product showed both to be low-barrier processes (SI). Therefore, we could not unambiguously rule out or confirm any of the routes.
Based on these preliminary data, we next investigated how the different alkyl groups would affect the reactivity, solubility, and stability of the triazinium ions. Methylated and ethylated derivatives are synthetically accessible via alkylation by commercial methyl- and ethyl-trifluoromethanesulfonates.19b For preparation of iso-propyl (iPr) and tert-butyl (tBu) analogs, we generated the corresponding triflates in situ from the respective alcohols and triflic acid anhydride in the presence of pyridine (SI).
Second order rate constants for all derivatives were determined in a CH3CN/H2O (1 : 1) mixture at room temperature using an excess of endo-BCN. These measurements showed that the reactivity decreased, while the size of the alkyl substituent in the series Me vs Et vs iPr increased (Figure 4A and Table S6 in the SI).
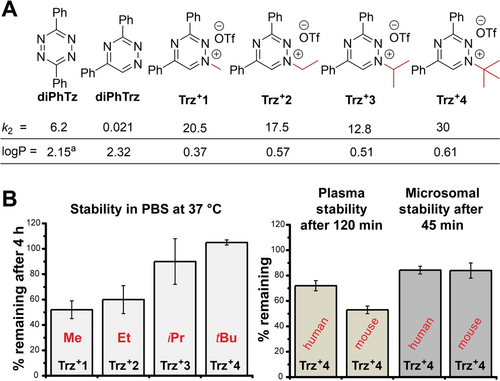
(A) Second-order rate constants (in M−1 s−1) for the reactions of diPhTz, diPhTrz, and differently alkylated triazinium salts with endo-BCN determined in a CH3CN/H2O (1 : 1) mixture at room temperature; experimentally determined logP values are also given. aThis logP value was calculated using StarDrop software due to very low solubility in the water/octanol mixture. (B) Stability of differently alkylated triaziniums in PBS at 37 °C and stability of Trz+4 in the plasma and microsomes of humans or mice.
Surprisingly, Trz+4 containing the bulky tBu group was found to be most reactive. Since it is also the most lipophilic derivative, the increase in reactivity may be attributed, at least in part, to the increased hydrophobicity effect.21 Alternatively, dispersion forces could contribute to this unusual structure–reactivity relationship.22 Interestingly, all triazinium salts proved more reactive than diPhTz in reaction with endo-BCN. This was also confirmed in a competition experiment, where an equimolar amount of Trz+4 and diPhTz was treated with one equivalent of endo-BCN. This experiment revealed that 3.4-times more of the triazinium click product was formed in the reaction mixture (Figure S15). The logP values increased with the steric bulk of the alkyl group, but fell well below those of the corresponding diPhTrz or diPhTz (Figure 4A). These data confirm the enhanced water solubility of the triazinium compounds. Further calculated and experimental logP values can be found in the SI.
During initial experiments, we noted that the solvent had a significant effect on the stability of the compounds. While all derivatives were stable for 4 hours in water (Figure S17), Me-, Et-, and iPr-substituted triaziniums partially decomposed over the course of 4 hours in phosphate-buffered saline (PBS) when incubated at 37 °C (Figure 4B). Interestingly, the major decomposition product was identified as the corresponding de-alkylated parent diPhTrz (Figure S18). The only exception was the tBu analog, which was perfectly stable in PBS. In fact, ca. 80 % and 70 % of Trz+4 remained intact even in full cell growth medium (Dulbecco's Modified Eagle Medium (DMEM) containing 10 % fetal bovine serum, FBS) when incubated at 37 °C for 24 and 48 hours, respectively (Figure S19). Additional plasma and microsomal stability (human and mouse) measurements corroborated the superior stability of the compound (Figure 4B). Dealkylation is probably not the main metabolic pathway of Trz+4 degradation even in living cells (Figure S20A and S21). Our data indicate that the compounds’ decomposition possibly involves formation of various species (Figure S22).
To study the reactivity of Trz+4 in more detail, we performed the ligation with endo-BCN in the presence or absence of reduced glutathione. NMR analysis of the crude mixtures revealed that in addition to tert-butanol, other tBu species were formed in the reaction (Figure S3). Subsequent attempts to identify this compound ruled out several potential candidates such as tBu amine (protonated or not), tBu hydroperoxide or tBu ether. The formation of this compound is not related to the presence of glutathione since the same product is formed in its absence. Furthermore, LC–MS analysis of the reaction mixture from the NMR tube confirmed clear formation of the desired ligation product 1 (Figure S4). Currently, the identity of this compound remains unclear.
Our initial t-butylation procedure used for preparing Trz+4 based on alkylation of diPhTrz by in situ-generated tBu triflate proved poorly reproducible. To ensure synthetic access to the compounds, we optimized this crucial step by investigating a number of reaction conditions and different alkylating agents (SI). This led to the identification of isobutene, which in the presence of triflic acid in DCM led to clean and regioselective alkylation at nitrogen N1 in a 95 % yield (Figure 5A). Importantly, when the alkylation reaction was successfully performed on a larger scale (2.5 g), the product was still isolated in an 80 % yield (SI).
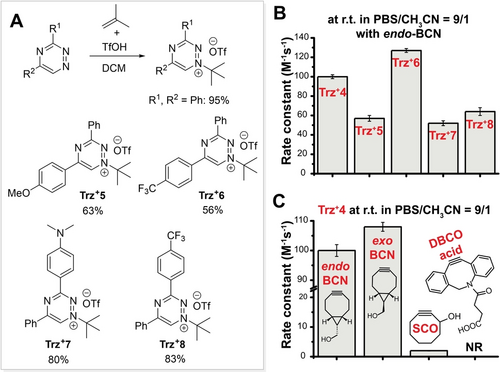
(A) Optimized t-butylation of 1,2,4-triazines using isobutene and triflic acid was used for the synthesis of a series of triazinium triflates (yields of isolated compounds are shown). (B) Graph shows second-order rate constants of reactions between differently substituted triaziniums and endo-BCN. (C) Graph shows second-order rate constants for reactions between Trz+4 and various dienophiles. r.t.=room temperature, NR=no reaction.
After successfully establishing synthetic access to the compounds, we next studied the effect of different counterions on the solubility of di-phenyl-substituted t-butylated triazinium salts. Solubility in PBS at pH=7.4 was around 90 μM and is similar for CF3SO3−, CF3COO− and Cl− counterions despite the different logP values of the triazinium salts (Table S11 in the SI). Surprisingly, we observed differences in the reactivity of triaziniums bearing different counterions, of which the Trz+4 (CF3SO3−) salt was the most reactive (Table S7 in the SI).
Using the optimized alkylation procedure, we prepared a small series of Trz+s bearing electron-withdrawing or -donating groups (Trz+5–Trz+8) at positions 3 or 5 of the phenyl ring to investigate the influence of substituents on the click reaction rate with endo-BCN (Figure 5A and 5B). As expected, introducing an electron donating methoxyphenyl group (Trz+5) at position 5 resulted in deceleration relative to diphenyl-substituted Trz+4 (57 M−1 s−1 vs 100 M−1 s−1). By contrast, the presence of an electron-withdrawing CF3-phenyl group at the same position increased the reactivity of Trz+6 (127 M−1 s−1).
For comparison, the rate constant of the reaction of 3,6-di-2-pyridyl-1,2,4,5-tetrazine (diPyTz) with endo-BCN is 540 M−1 s−1, which is about four-times higher than that of Trz+6 (Figure S16 and Table S8). On the other hand, Trz+6 proved stable (>80 % remained in the solution after 24 hours at 37 °C) in DMEM containing 10 % FBS even in the presence of 10 mM reduced glutathione. In contrast, only about 30 % of diPyTz remained intact after this time (Figure S19 and S20 C). Modification of the phenyl ring at position 3 of the triazinium ring by electronically different substituents decreased the reactivity and yielded rate constants of 52 M−1 s−1 and 64 M−1 s−1 for Trz+7 and Trz+8, respectively. Trz+7 is perfectly stable within 24 hours in full cell growth medium, while about 20 % of Trz+8 decomposed under these conditions (Figure S19).
To test the potential for Trz+s to be used in combination with other dienophiles, we measured the reaction kinetics of Trz+4 with exo-BCN isomer, the less strained cyclooct-2-yn-1-ol (SCO) and dibenzocyclooctyne acid (DBCO-acid) (Figure 5C). The difference in reactivity between the two BCN isomers with Trz+4 was small (100 M−1 s−1 vs 108 M−1 s−1). In contrast, SCO reacted two orders of magnitude slower (2 M−1 s−1), which is in the range of reactivity exhibited by, e.g., tetrazines with norbornenes.8b Interestingly, we isolated a single regioisomer from the reaction of SCO with Trz+4. This offers the possibility of preparing defined ligation products using this dienophile. On the other hand, we did not observe any reaction of Trz+4 with DBCO-acid. This might be attributed to increased steric demand in the transition state, as in the case of 1,2,4,5-tetrazines.23 Our initial experiments using trans-cyclooctene as the dienophile in the reaction revealed an unusual product formation. Therefore, the reactivity of this and other strained alkenes such as derivatives of norbornenes or cyclopropenes is the subject of an ongoing study.
Various mutually orthogonal biocompatible reactions are becoming increasingly popular as they provide an opportunity to analyze complex biological events simultaneously.24 Given that Trz+4 did not react with DBCO-acid, we decided to explore the possibility of using triazinium ligation in combination with the SPAAC reaction (Figure 6). In the first experiment, we mixed equivalent amounts of 3-azido-coumarin (Az-Coum), Trz+4, and DBCO-acid in a CH3CN/H2O (1 : 1) mixture and incubated the reagents for 1 hour at room temperature. After this time, HPLC-MS analysis showed only a selective SPAAC reaction between Az-Coum and DBCO-acid, indicating that neither azide nor DBCO reacted with the triazinium (Figure S25). To explore the selectivity further, we mixed an equimolar amount of Az-Coum and Trz+4 with one equivalent of endo-BCN. In this case, the azide and triazinium competed for the strained alkyne. Due to the much higher reactivity of endo-BCN with Trz+4 compared with the azide, HPLC-MS recorded a signal corresponding almost exclusively to the triazinium click ligation product as early as in 30 min after the experiment. Only traces of the corresponding SPAAC product arising from the reaction of Az-Coum with endo-BCN were detected in the chromatogram (Figure S26 in the SI). Finally, we performed the experiment with all four reagents by combining pre-mixed solutions of Az-Coum and Trz+4 with endo-BCN and DBCO-acid. After 30 min, HPLC-MS analysis confirmed a selective reaction of Trz+4 with endo-BCN and of DBCO-acid with Az-Coum. Only traces of the SPAAC product between Az-Coum and endo-BCN were detected (Figure 6 and S27).These data indicate that triazinium ligation is orthogonal to the popular SPAAC reaction, making this conjugation a valuable addition to the repertoire of mutually orthogonal biocompatible reactions.
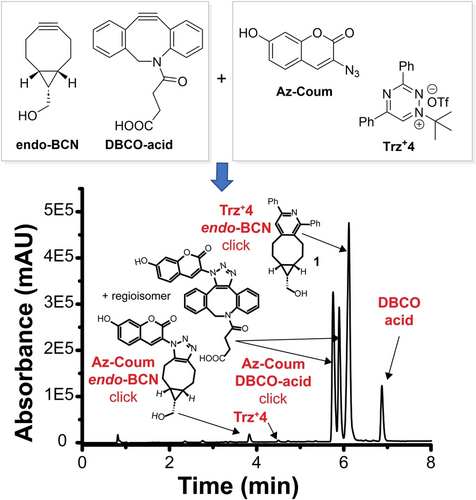
Mutually orthogonal click reaction experiment. An equimolar amount of endo-BCN and DBCO-acid was combined with an equimolar amount of Az-Coum and Trz+4 (all at 1 mM final concentration) in a CH3CN/H2O (1 : 1) mixture at room temperature. HPLC-MS analysis of the crude reaction mixture performed after 30 min revealed highly selective formation of SPAAC and triazinium click ligation products. Additional experiments can be found in the SI.
Labeling and modification of biomolecules are important applications of bioconjugations. To investigate the extent of selectivity and efficiency of the triazinium ligation when performed on biomolecules, we first prepared a BCN-containing peptide (BCN-peptide)25 and mixed it with Trz+4. The reagents were incubated for 30 min in a CH3CN/H2O (1 : 1) mixture at room temperature. The reaction proceeded cleanly and selectively, and the expected click peptide was formed in full conversion (Figure 7A and S28).
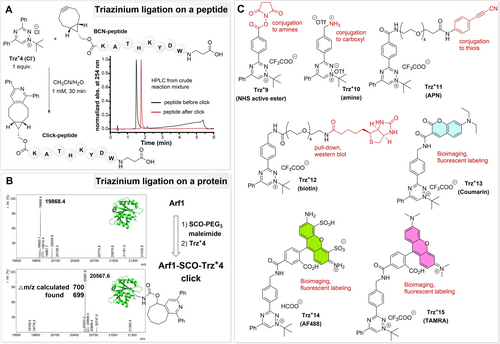
Triazinium ligation was used to label (A) model peptide and (B) the protein Arf1. (C) Examples of triazinium derivatives bearing different functional moieties useful for various synthetic and biological applications.
To study the new triazinium ligation on a protein, we performed the reaction with ADP-ribosylation factor 1 (Arf1).26 First, the single cysteine residue of Arf1 was modified with the commercial SCO-PEG3-maleimide to yield Arf1-SCO (SI). After gel filtration, we added Trz+4 (10 equiv.) to Arf1-SCO and incubated the mixture for 2 hours at room temperature. After that, intact protein mass analysis of the reaction mixture revealed successful formation of the click-labeled product without any traces of the starting protein (Figure 7B). Importantly, we did not observe any modification or side reactions in the control experiment, where unmodified Arf1 protein was incubated under the same conditions with Trz+4 (Figure S29 in the SI). This result indicates that the reaction depends on the presence of the strained alkyne and that it is selective even in the presence of other functional groups in the side chains of amino acids. These experiments highlight the favorable selectivity and efficiency of the triazinium ligation and its application potential for labeling biomolecules.
To demonstrate that the triazinium click group can be embedded into structurally more complex molecules, we prepared a series of derivatives bearing various functional moieties (Figure 7C). These included an N-hydroxysuccinimide (NHS) active ester (Trz+9) for modification of amino group-containing molecules (e.g., small molecules, peptides, or proteins), an amino group (Trz+10) for peptide couplings, arylpropionitrile (APN) for conjugation to cysteine residues (Trz+11), biotin for pull-down or western blot analysis (Trz+12), and various dye conjugates for bioimaging applications (Trz+13–15). The latter derivatives are synthetically accessible by late-stage functionalization of Trz+9 and Trz+10 (SI).
Labeling and visualization of molecules in living cells are among the most challenging applications of bioorthogonal reactions.2a Accordingly, we analyzed the triazinium ligation in this complex environment. To compare the new reagents with the more established 1,2,4,5-tetrazines, we used a set of analogous compounds that differ only in the heterodiene part. In the first experiment, we performed the reaction on living cells treated with BCN-modified concanavalin A (BCN-ConA), a lectin with high specificity for α-d-mannose and α-d-glucose residues.27 In the control experiment, cells were treated with the same amount of the labeling reagent, but without the lectin. U2OS osteosarcoma cancer cells pretreated with BCN-ConA were washed and incubated with 10 μM Trz+14 or the analogous MeTz-AF488 for 1 hour. After washing, cells were inspected on a confocal microscope. Under these conditions, both reagents provided a clean labeling pattern on the cell surface (Figure 8A and 8C and Figure S30). Time-lapse experiment showed that visible signal formed on the cell surface already after 15 min of incubation with Trz+14 (Figure S38). We did not observe any labeling in control experiments when cells were pre-treated only with the labeling reagent (Figure 8B and 8D). These data show that the new triazinium ligation is comparable to the popular tetrazine ligation in enabling efficient labeling of BCN-modified proteins on living cells.
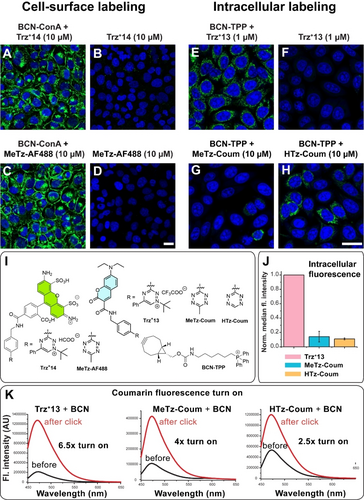
Comparison of triazinium and tetrazine ligations in bioimaging experiments. Cell surface labeling: living U2OS cells were treated with BCN-ConA and subsequently with Trz+14 or MeTz-AF488 (10 μM for 1 hour). Control cells were treated in the same way but only with Trz+14 or MeTz-AF488, respectively. Cells were washed with cell medium before imaging. (A) Labeling with Trz+14 and (B) the corresponding control experiment. (C) Labeling with MeTz-AF488 and (D) the corresponding control experiment. The click labeling signal is shown in green. Cell nuclei were stained with Hoechst 33342 dye shown in blue. Intracellular labeling: living HeLa cells were treated with BCN-TPP (5 μM for 10 min) and subsequently for 30 min with (E) Trz+13 (1 μM), (F) only with Trz+13 (1 μM), (G) with MeTz-Coum (10 μM) or (H) with HTz-Coum (10 μM). Cells were washed twice before imaging. Further experiments performed at different concentrations and control experiments can be found in the SI. Cell nuclei were stained with DRAQ5 dye shown in blue. Scale bar is 25 μm. (I) Structures of the reagents. (J) Data from flow cytometry of cells treated with MeTz-Coum, HTz-Coum and Trz+13 (1 μM for 30 min) demonstrating the enhanced cell permeability of the triazinium reagent. Data were corrected for different fluorescence intensities of the fluorophores and normalized (Figure S32) (K) Fluorescence spectra of coumarin reagents before and after the reaction with endo-BCN highlight the fluorogenic properties of the compounds.
To further evaluate the triazinium reagents, we performed the reaction inside living cells. In this experiment, we used a BCN-triphenylphosphonium conjugate (BCN-TPP), which we previously employed in intracellular fluorescence turn-on labeling studies.28 The TPP moiety is known to target small molecules to mitochondria thus ensuring the construct remains inside the cells.29 We compared the new triazinium reagents with the corresponding tetrazines in this case as well. Living HeLa cells were treated with BCN-TPP for 10 min and subsequently washed to remove any extracellular compound. Next, the click reagents conjugated to the cell-permeable coumarin dye (Trz+13 and MeTz-Coum) were added to the cells at a low 1 μM concentration and were incubated for 30 min. The cells were washed and inspected on a confocal microscope. In contrast to Trz+13, which showed clear labeling inside the cells (Figure 8E), we did not observe any labeling with the corresponding tetrazine reagent at this concentration. Increasing the concentration 5-fold did not improve the results (Figure S32). Finally, 10 μM of MeTz-Coum yielded weak, but visible signal inside the cells (Figure 8G). We assumed the lower reactivity of the methyl tetrazine was responsible for the reduced intensity of the labeling.30 Therefore, we performed the same experiment, but this time used the corresponding coumarin probe containing the more reactive H-tetrazine (HTz-Coum). Surprisingly, even the use of HTz-Coum did not result in efficient labeling in cells at 1 μM concentration (Figure S32). However, increasing the concentration of HTz-Coum to 5 μM and then to 10 μM led to the formation of a visible signal inside the cells (Figure 8H). We also quantified the labeling efficiency of the individual compounds by flow cytometry, which confirmed the data obtained from confocal microscopy (Figure S33).
To rule out the possibility that the observed enhanced labeling was limited to the mitochondrial BCN-TPP probe (Trz+13 is located also in other cellular compartments, Figure S31), we performed analogous experiments with BCN-PEG3-amine with affinity for lysosomes.31 These results proved similar, corroborating the higher labeling efficacy of Trz+13 at 1 μM concentration in comparison with MeTz-Coum and HTz-Coum (Figure S34, S36 and S37). Additional time-lapse experiments showed that the intracellular labeling reactions yield visible signal already after 1 min of incubation with Trz+13 (Figure S39), which highlights the efficiency of the reaction inside cells.
Unsurprisingly, the reactivity of bioorthogonal reagents is not the sole determinant of their performance inside living cells. We and others have observed that cell permeability and stability of compounds are equally important parameters.31, 32 To explain the enhanced intracellular labeling observed for the triazinium salt, we incubated cells with all three compounds: Trz+13, MeTz-Coum and HTz-Coum. We then briefly washed the cells, and quantified intracellular fluorescence intensity by flow cytometry. This analysis revealed that larger amount of the charged triazinium was present inside the cells compared with the tetrazines (Figure 8J). We also quantified the amount of the compounds present inside the cells by mass spectrometry. This experiment confirmed that more of Trz+13 enters the cells (Figure S40D). These results can be attributed to the enhanced cell permeability of compound Trz+13, which differed from the respective tetrazines only in the heterodiene part.
We also speculated whether the potential fluorogenic properties of the probes would contribute to the observed differences in labeling. Based on measurements of the fluorescence spectra of the coumarin-containing probes before and after the reaction with endo-BCN all three compounds exhibited fluorogenic properties. However, there were no significant differences (Figure 8K and S44). Surprisingly, we observed turn-on in fluorescence even after the reaction of Trz+13 with the dienophile, which suggests that triazinium salts could serve as a new fluorescence quenching moiety. These properties are currently the subject of an ongoing study in our lab. Based on the obtained data, we conclude that the increased labeling efficiency inside living cells is mainly due to the increased cellular permeability of the positively charged triaziniums.
We also studied the toxicity of the triazinium probes on four cell lines (Figure S45 and Table S12). These experiments revealed IC50 values in the lower micromolar range, with the CF3SO3− salt being the least toxic. All investigated compounds were in most cases more toxic to cancer cell lines (HepG2, HeLa and U2OS) than they were to the non-tumor human dermal fibroblasts (NHDF). Due to the low concentration and short incubation time, which are sufficient to achieve an efficient intracellular labeling reaction, we believe that triazinium salts can be considered useful for applications involving living cells as well. However, potential toxicity should be carefully considered and evaluated in cases where longer incubation times with the compounds will be required. Clearly, additional structural optimizations will be needed to further improve the toxicity profile of these charged heterodienes.
Conclusion
In this work, we introduce a new bioorthogonal conjugation, triazinium ligation, which is based on the reaction of N1-alkyl 1,2,4-triazinium salts with strained alkynes. The key triazinium reagents are readily accessible via optimized alkylation of 1,2,4-triazines, enabling access to derivatives with tunable reaction rates. Alkylation accelerates the reaction by three orders of magnitude compared with the parent triazines. The enhanced reactivity of the salts is caused by the lower relative free energy of the corresponding transition state, as revealed by DFT calculations. From among the alkylated derivatives, tert-butylation was the modification of choice as the corresponding triazinium salts proved both, highly reactive and stable in a biological milieu. The triazinium ligation is orthogonal to the strain-promoted azide-alkyne cycloaddition, which should allow these two reactions to be performed on a single system. We demonstrate that this new conjugation can be used for clean and efficient modification of biomolecules such as peptides and proteins. According to our comparative cell-labeling study, the increased cell permeability of the positively charged triazinium salts enhanced the efficiency of intracellular labeling compared with tetrazine derivatives bearing the same substituents. Based on the data presented here, we expect triazinium ligation to become a valuable addition to existing bioconjugations, and that this chemical reaction will further expand the application potential of bioorthogonal transformations in chemical biology and beyond.
Supporting Information
Synthetic procedures, characterization data of all new compounds, kinetic measurements, compound stability studies, additional cellular experiments and cytotoxicity measurements. This material is available in the Supporting Information.
Acknowledgments
This work was supported by the Academy of Sciences of the Czech Republic (RVO: 61388963), the Czech Science Foundation (P207/20-30494L) and the National Institute for Research of Metabolic and Cardiovascular Diseases (Programme EXCELES, ID Project No. LX22NPO5104)—Funded by the European Union—Next Generation EU. V. S. is grateful for support from the IOCB postdoctoral fellowship. We wish to thank Prof. Pavel Kočovský for critical reading of the manuscript, Michael FitzGerald for language corrections, the Department of Mass Spectrometry at IOCB for measurement of the intact protein masses, the group of Biochemical pharmacology at IOCB, and Jana Günterová for help with the flow cytometry analysis.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.