Closed-Loop Recycling of Vinylogous Urethane Vitrimers
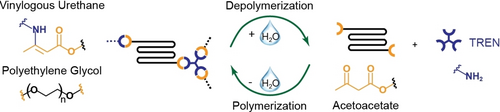
Graphical Abstract
The closed-loop recycling of vinylogous urethane vitrimers made from acetoacetate-terminated polyethyleneglycol and tris(2-aminoethyl)amine is presented. Treatment with water allows the selective and quantitative recovery of the monomers, even from mixed waste streams. The modular synthetic approach allows the synthesis of materials that cover a broad range of mechanical properties and that are easy to reprocess.
Abstract
Devising energy-efficient strategies for the depolymerization of plastics and the recovery of their structural components in high yield and purity is key to a circular plastics economy. Here, we report a case study in which we demonstrate that vinylogous urethane (VU) vitrimers synthesized from bis-polyethylene glycol acetoacetates (aPEG) and tris(2-aminoethyl)amine can be degraded by water at moderate temperature with almost quantitative recovery (≈98 %) of aPEG. The rate of depolymerization can be controlled by the temperature, amount of water, molecular weight of aPEG, and composition of the starting material. These last two parameters also allow one to tailor the mechanical properties of the final materials, and this was used to access soft, tough, and brittle vitrimers, respectively. The straightforward preparation and depolymerization of the aPEG-based VU vitrimers are interesting elements for the design of polymer materials with enhanced closed-loop recycling characteristics.
Introduction
Since the development of Bakelite in 1907,1 polymers have become indispensable to our society. However, the remarkable growth of the world's plastic production is unsustainable2 and makes it necessary to develop effective strategies for end-of-life scenarios of these materials that go beyond mechanical recycling and incineration.3 One of the most promising new approaches to replace traditional polymers consists in the creation of polymers comprising dynamic covalent bonds, i.e., covalent linkages that can dynamically form, dissociate, or exchange with one another.4
Early reports on polymers featuring dynamic covalent bonds date back to the 1940s, when Green and Tobolsky postulated that disulfide cross-links are reversible, and enable non-degradative bond rearrangements and stress relaxation.4 However, dynamic covalent chemistry, i.e., the chemistry of covalent bonds undergoing dynamic exchange, became a firmly established field only in the late 1990s/early 2000s.5 This “supramolecular chemistry at the covalent level“, as defined by Rowan and co-workers, proved to be relevant in the preparation of dynamic small molecules and macromolecules.5 Further elaborating on the exploitation of dynamic linkages in polymer materials, Leibler et al. reported a major breakthrough with the development of vitrimers in 2011.6 These polymeric materials contain dynamic covalent linkages that undergo chemical exchange reactions via an associative mechanism.7 This feature allows the thermal reprocessing and recycling of polymer networks—which is otherwise only possible for thermoplastics—and it simultaneously provides the material with chemo-mechanical robustness during the material's service time, on account of the cross-linked structure—as in thermosets.8 Leibler's seminal report leveraged esters and transesterification processes,6 but the library of dynamic motifs (and associative exchange reactions) has significantly expanded since then, with copious reports on imines (transiminations),9 diketoenamines and vinylogous urethanes (transaminations),10 acetals (transacetalations),11 thioureas (transcarbamoylations),12 alkenes (olefin metathesis),13 dioxaborolanes (metathesis),7 and silyl ethers (exchanges).14 A clear advantage of most of these materials in comparison to conventional thermosets is that they can be reprocessed or recycled several times by thermal treatment, which in principle should greatly reduce the need for virgin plastics and reduce their end-of-cycle environmental impact.10a, 10b, 15 However, the high temperatures necessary for the activation of the dynamic bonds can lead to some chemical degradation, which in turn impacts the physical properties and can cause unappealing discoloration after multiple reprocessing cycles. These problems can be avoided by depolymerization of the vitrimers under mild conditions, recovery of the constituents of the networks, and their subsequent repolymerization (i.e., closed-loop recycling). Previous reports have highlighted that the complete recovery of the starting monomers was not always possible, possibly due to challenging separation of the materials’ components derived from the depolymerization process and the delicate interplay between kinetics and thermodynamics that often lead to non-exhaustive depolymerization processes.5, 16 Nevertheless, the illustrious examples of the closed-loop recycling of diketoenamine vitrimers from the Helms lab10a and the alkyl-polycyanurate thermosets reported by the Zhang group15 show that the exhaustive depolymerization of polymer networks comprising dynamic covalent bonds is a priori feasible.
Among the numerous vitrimers reported thus far, the vinylogous urethanes (VU) pioneered by the Du Prez group10b have shown remarkable potential for applications, on account of their versatility, ease of synthesis, and high processability. Selected examples that showcase such broad applicability are VU materials used for 3D printing,17 electrochemistry (as electrolytes),18 antibacterial coatings,19 shape memory materials,20 and drug delivery.21 Researchers have also rapidly developed an exquisite knowledge of the molecular-level processes of VU-based systems, which has been instrumental to achieve a high level of control over the vitrimeric behavior and reprocessability.10c, 22 However, to our best knowledge, investigations that tackle the closed-loop recycling of this rapidly growing class of vitrimers are lacking. With this in mind, we set out to create VU vitrimers that can be completely depolymerized in the presence of water and at ambient temperature. Although polymers that depolymerize/degrade upon exposure to neutral water might not have broad applicability, the materials selected for this study represent an ideal testbed to investigate fundamental aspects, and we hope the data will catalyze the further development of novel and technologically relevant vitrimers with closed-loop recycling capability.
Our materials design concept builds on the chemical equilibrium of the formation of VU from acetoacetate moieties and amines, which produces water as byproduct (Scheme 1). Following the Le Chatelier principle, it should consequently be possible to depolymerize VU vitrimers into the parent monomers by treatment with (an excessive amount of) water. The concept is simple and chemically sound, but it has not been reflected in the literature of VU vitrimers, perhaps because these materials often contain hydrophobic components that do not allow water to swell the polymers in sufficiently high concentration. This prevents complete depolymerization by limiting the concentration of water. We hypothesized that realizing VU vitrimers comprising polyethylene glycol (PEG) as major component, and tris(2-aminoethyl)amine (TREN) as cross-linker, would favor the depolymerization and closed-loop recycling of the network upon treatment with water. Here, we show that this is indeed the case and that this design approach allows one to fine-tune the thermomechanical properties and depolymerization rate of the resulting PEG-based VU networks. We first discuss the synthesis and mechanical properties of the new VU vitrimers, and then address the recovery of the PEG constituents after the depolymerization of the vitrimers. This is then followed by a demonstration of the reprocessing of the PEG-based VU networks either by heat-induced transamination processes or a water-mediated depolymerization-repolymerization scheme.
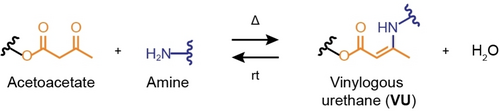
Chemical equilibrium involving an acetoacetate motif and an amine, leading to the formation of a vinylogous urethane (VU) linkage, and water as byproduct. The formation of the VU bond is favored by heat and removal of water (forward reaction), while the addition of water shifts the equilibrium towards the starting materials (backward reaction).
Results and Discussion
Synthesis, Characterization, and Mechanical Properties of the Vinylogous Urethane Vitrimers
The here-investigated polymers were all based on bis(acetoacetate)-terminated poly(ethyleneglycol) building blocks (aPEGx; Figure 1a). The subscript x indicates the number-average molecular weight (Mn) of the parent PEGx. Different aPEGx grades were prepared from commercially available, hydroxy-terminated polyethylene glycols (PEGx) with an Mn of 1000 (PEG1k), 2000 (PEG2k), 3000 (PEG3k), 10000 (PEG10k), or 35000 (PEG35k) g ⋅ mol−1 in transesterification reactions with tert-butyl acetoacetate (see page S4 of the Supporting Information for details). Adapting a recently reported procedure,18 the reactions were carried out in the presence of an excess of tert-butyl acetoacetate (20 eq. for PEG1k, PEG2k, and PEG3k; 50 eq. for PEG10k and PEG35k). The yield of these reactions ranged from 90 % to 94 %, while the extent of end-group functionalization varied from 92 % to 100 %, as determined by the integration of the signals in the 1H NMR spectra of the purified aPEGx (Figures S1–5; calculations on page S4–5). FT-IR spectra of the aPEGx derivatives reveal the disappearance of the broad band at 3450 cm−1, which is assigned to the −OH end groups of the PEGx, and the appearance of two peaks at 1716 and 1741 cm−1, which are ascribed to the C=O stretching vibrations of the acetoacetates introduced as chain-ends of the aPEGx (Figure S9).
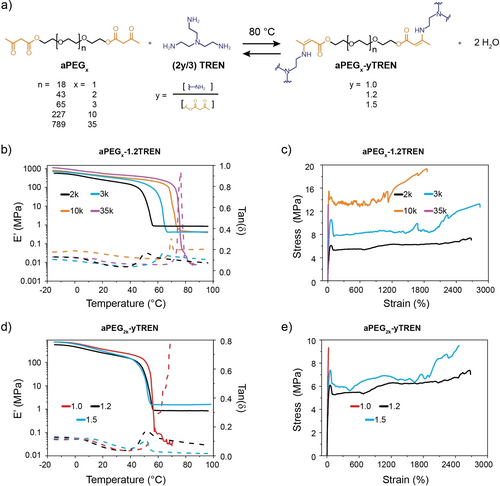
(a) Synthesis of aPEGx-yTREN by the reaction of bis(acetoacetate)-terminated poly(ethyleneglycol) (aPEGx) and tris(2-aminoethyl) amine (TREN). Parameters x and y indicate the number-average molecular weight (Mn) of the PEGx segment in 1000 g ⋅ mol−1 and the molar ratio of amine to acetoacetate functional groups, respectively. (b, d) DMA traces showing the storage modulus (E′) (solid lines) and tan δ (dashed lines) of (b) the aPEGx-1.2TREN series and (d) the aPEG2k-yTREN series as a function of temperature. (c,e) Stress-strain curves of (c) the aPEGx-1.2TREN series and (e) the aPEG2k-yTREN series.
Polymer networks comprising VU linkages (aPEGx-yTREN) were synthesized by reacting the various aPEGx with tris(2-aminoethyl)amine (TREN). These condensation reactions involve the acetoacetate end groups of the aPEGx and the amine functions of TREN; they proceed smoothly at 80 °C and afford vinylogous urethanes (VUs) and water as byproduct (Figure 1a). We synthesized several aPEGx-yTREN grades by varying the Mn of aPEGx and the molar ratio of amine to acetoacetate functional groups (y), which can be easily controlled by the feed of TREN and aPEGx (Figure 1a; Table S1). aPEGx-yTREN grades with y=1.0 (i.e., an equimolar concentration of amine and acetoacetate groups), 1.2 (i.e., a 20 % mol excess of amines), and 1.5 (i.e., a 50 % mol excess of amines) were explored (Figure 1a; Table S1). aPEGx-yTREN films were prepared by mixing aPEGx and TREN in DMF, casting the resulting solutions into Teflon molds, heating the mixtures overnight to 80 °C, and subsequently vacuum drying the resulting films for 12 h at 80 °C (details on page S9). The materials thus prepared were analyzed by FT-IR spectroscopy, which reveals the disappearance of the acetoacetates peaks at 1716 and 1741 cm−1, and the appearance of two new vibrational bands at 1604 and 1648 cm−1 (Figures S9, S10b), which indicate the formation of the vinylogous urethane bonds.10b, 18 The intensity of these new signals decreases (at constant y) as the Mn of aPEGx is increased, reflecting that the cross-link density is reduced (Figure S10b). We also investigated the swelling ratio and gel fraction of the aPEGx-yTREN networks by immersing the films in DMF (Figure S11), in which both aPEGx and TREN are readily soluble. Within the aPEGx-1.2TREN series, the swelling ratio increases from 500 % to 1520 %, and the gel fraction decreases from 94 % to 66 %, as the Mn of aPEGx is increased from 1 to 10 kg ⋅ mol−1 (Figure S11a). Increasing the feed of TREN (i.e., a higher y value) and keeping aPEGx the same causes a slight decrease of the swelling ratio, while the gel fraction remains practically the same (Figure S11b). These results reflect that the parameters x and y allow one to control the cross-link density and thereby the swelling characteristics of the aPEGx-yTREN networks. aPEG2k-1.0TREN and aPEG35k-1.2TREN fully dissolved in DMF, suggesting that a very low TREN feed or a very high Mn of aPEGx prevents the formation of cross-linked networks.
The thermal properties of the various PEGx, aPEGx, and aPEGx-yTREN networks were studied by differential scanning calorimetry (DSC) (Figure S12). The DSC heating traces of all polymers, except aPEG1k-1.2TREN, show endothermic peaks at temperatures between 38 and 61 °C, which are associated with the melting of crystalline domains formed by the PEG segments.22 The melting temperature (Tm) increases with the Mn of aPEGx (Table S2). For any given value of x, the Tm, and degree of crystallization (χc) decrease in the order of PEG>aPEGx>aPEGx-yTREN (Figures S12a–e, Table S2). For instance, the Tm, and χc values of PEG2k, aPEG2k, and aPEG2k-1.2TREN are 52 °C and 83 %, 41 °C and 70 %, and 38 °C and 38 %, while aPEG1k-1.2TREN is fully amorphous. The enthalpy of crystallization (ΔHc) shows the same trend (Table S2). Similar behavior was observed for the other aPEGx-yTREN series investigated (Figures S12a–e). Increasing the y value resulted in a small decrease of Tm, χc, and ΔHc, as exemplified by the data for the aPEG2k-yTREN series in Table S2, in which the Tm, χc, and ΔHc dropped from 39 °C, 41 %, and 81 ⋅ J ⋅ g−1 (y=1.0), to 38 °C, 38 %, and 74 J ⋅ g−1 (y=1.2), and 35 °C, 34 %, and 69 J ⋅ g−1 (y=1.5).
We next studied the thermomechanical properties of aPEGx-yTREN films by means of dynamic mechanical analysis (DMA) (Figures 1b, 1d). All DMA traces of aPEGx-1.2TREN (x≥2k) feature a slanted plateau regime in which the storage modulus E′ adopts values between 1110 and 570 MPa. A gradual reduction of E′ with temperature can be observed, and the E′ trace shifts to higher values as the Mn and χc are increased. The DMA traces all show a sharp drop of E′ at a temperature that marks the melting of the crystalline PEG domains. The corresponding maxima of the tan delta (tan(δ)) plots are in good agreement with the melting temperatures observed by DSC (see above). In the case of aPEG2-1.2TREN, aPEG3-1.2TREN, and aPEG10-1.2TREN, the DMA traces show a rubbery plateau, confirming the formation of cross-linked architectures. In this regime, aPEG2k-1.2TREN, which features the highest cross-link density, displays the highest E′, whereas no differences can be observed for aPEG3k-1.2TREN, and aPEG10k-1.2TREN. By contrast, aPEG35k-1.2TREN fails upon melting, reflecting that the cross-link density in this material is very low. The DMA trace of aPEG1k-1.2TREN shows no obvious thermal transitions in the temperature range explored (−20 °C to 100 °C; Figure S13a), consistent with the absence of crystalline PEG domains, as evidenced by DSC data (Figure S12a). Figure 1d, which shows the DMA traces of the aPEG2k-yTREN series, confirms that changing the TREN content has only a minor effect on Tm, as reflected by the DSC data. However, a pronounced effect is observed above Tm, where the increased cross-link density bestows aPEG2k-1.5TREN with a higher E′ (1.5 MPa) than aPEG2k-1.2TREN (0.9 MPa), and the absence of cross-links causes aPEG2k-1.0TREN to fail upon reaching Tm.
The mechanical properties of films of the aPEGx-1.2TREN series were further investigated by tensile testing (Figure 1c; Figure S14, Table S3). The stress-strain curves of aPEG2k-1.2TREN, aPEG3k-1.2TREN, and aPEG10k-1.2TREN all display the typical behavior of semicrystalline polymers, with an initial elastic regime, yield points at 30–90 % strain, and yielding deformation with modest or no strain hardening (Figure S14b). The yield stress and stress at break increase with the Mn of aPEGx from 6.1 MPa and 7.0 MPa for aPEG2k-1.2TREN to 15.5 MPa and 19.3 MPa for aPEG10k-1.2TREN, and all three polymers show plastic deformation with a strain at break of more than 1800 % (Figure 1c; Table S3; Supplementary Video S1). In stark contrast, aPEG1k-1.2TREN, in which the PEG segments do not crystallize, is an elastic material and displays much lower stress (0.9 MPa) and strain at break (98 %) values (Figure S13b). On the other hand, aPEG35k-1.2TREN is highly crystalline and shows brittle failure at a maximum stress of 7.5 MPa and low strain at break (7.5 %). The influence of the TREN content on the tensile properties is evident from the data shown in Figure 1e. aPEG2k-1.5TREN displays tensile properties that are very similar to those of aPEG2k-1.2TREN, but the increased cross-link density leads to a higher stress at break (Figure 1e; Figure S15, Table S4). Somewhat surprisingly, aPEG2k-1.0TREN shows brittle failure at a strain of 30 % and a stress of 9.3 MPa, which is higher than the yield stress observed for aPEG2k-1.2TREN and aPEG2k-1.5TREN (Figure S15b). We speculate that the low strain and high stress at break for aPEG2k-1.0TREN can be attributed to the low cross-link density induced by the low TREN feed in the synthesis and the high χc, respectively (Table S2).
Next, we tested the stability of the aPEGx-yTREN materials to oxidative conditions (for amino groups, i.e., high T and presence of air). Films of aPEG10k-1.2TREN were placed in an oven at 80 °C with constant air flow for 2 days. The aPEG10k-1.2TREN films thus treated were then subjected to tensile tests. The stress-strain curves recorded reveal a slight reduction in both stress and strain at break (21.1 MPa, 1180 %) compared to the original aPEG10k-1.2TREN (24.0 MPa, 1380 %) (Figure S16), which we attribute to the oxidation of (some) amino groups present in the material.
Because the aPEGx-yTREN VUs are based on hydrophilic PEG building blocks, their mechanical properties can be expected to depend on the relative humidity (RH) of the environment. To explore this, films of aPEG1k-1.2TREN and aPEG10k-1.2TREN were conditioned at 85 % RH for either 1 or 3 days. Subsequent tensile tests (Figure S17) show that the properties of aPEG10k-1.2TREN are hardly affected, whereas the stress and strain at break of aPEG1k-1.2TREN films drop from 0.9 MPa and 98 % to 0.6 MPa and 43 % after 1 day at 85 % RH and to 0.4 MPa and 29 % after 3 days at 85 % RH (Figure S17a). The different susceptibility to humidity is attributed to the crystalline nature of aPEG10k-1.2TREN (Figure S12d), which limits the uptake of moisture.23
Since responsiveness to moisture is normally not a desirable feature in polymeric materials, we also investigated aPTHF2k-1.2TREN (Figure 2), which is based on a hydrophobic building block made by end-capping poly(tetrahydrofuran) with an Mn of 2000 g ⋅ mol−1 with bisacetoacetate (aPTHF2k) and TREN (synthetic details on pages S5 and S10) to understand whether the moisture sensitivity could be suppressed. Gratifyingly, aPTHF2k-1.2TREN exhibits excellent stability in the presence of various organic solvents (such as THF, CHCl3, ethanol, DMF, and hexane), water, THF/H2O mixtures, and aqueous NaOH after 3 days at room temperature (Figure S18). More importantly, the mechanical properties of aPTHF2k-1.2TREN show no appreciable changes after 1 or 3 days of exposure to 85 % RH (Figure S19). Thus, the data show clearly that moisture sensitivity can be controlled via the crystallizability and polarity of the VU-terminated building block.
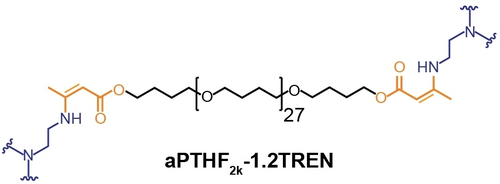
Chemical structure of aPTHF2k-1.2TREN.
Depolymerization and Recovery of the Monomers
As reflected by Figure 1a, the formation of vinylogous urethanes is a reversible process in which the position of the equilibrium can be shifted by the addition or removal of water. To test if the addition of water would lead to depolymerization of the VU-based polymers, we first investigated the hydrolysis of vinylogous urethane bonds using bis-(butyl vinylogous urethane)-terminated PEG (aPEG2k-Btl; Figure 3a), a model compound made by reacting aPEG2k and butylamine (Btl; page S19). The conversion of aPEG2k-Btl into aPEG2k and Btl was carried out by immersing 20 mg of aPEG2k-Btl in 700 μL of deuterated water (D2O) at 25 °C, 40 °C, and 60 °C (Figure 3a), and the progress of the reactions was monitored by following the evolution of the 1H NMR signals assigned in Figure 3a over time (Figure 3b; Figures S21b–d). Integration of the 1H NMR signals allowed us to quantify the degree of hydrolysis (Figure S21e). At 25 °C, the extent of hydrolysis of aPEG2k-Btl was ≈13 % after 30 min, increased to ≈36 % after 120 min, and reached a value of ≈88 % after 9 h (Figure 3b). When the reaction was carried out at 60 °C, ca. 93 % of aPEG2k-Btl were hydrolyzed within 1 h. Monitoring the consumption of aPEG2k-Btl in the presence of a large excess of D2O as a function of time and temperature (Figure S21e) allowed us to determine the pseudo-first-order kinetic rate constants (k) of the reaction (Figure S23, Table S5). An Arrhenius plot of ln(k) against 1/T affords an activation energy (Ea) of 61 kJ mol−1 for the process (Figure 3c).

(a) The hydrolysis of aPEG2k-Btl into aPEG2k and Btl; this reaction was carried out in D2O and at different temperatures. (b) Partial 1H NMR spectra recorded during the hydrolysis of aPEG2k-Btl at 25 °C; samples were taken at the times indicated. The signals used for the integration are assigned in Figure 3a. (c) Arrhenius plot for the hydrolysis of aPEG2k-Btl using an excess of D2O; the analysis affords an activation energy (Ea) of 61 kJ mol−1. (d) Scheme showing the hydrolysis of aPEG2k-1.2TREN into aPEG2k and TREN; this reaction was carried out in D2O and at different temperatures. The yellow box highlights protons m used for the determination of the relative depolymerization degree (RDD) in the 1H NMR experiments. (e) RDD for the depolymerization of aPEG2k-1.2TREN under formation of aPEG2k and TREN as a function of time at 20 °C, 40 °C, 60 °C, and 80 °C in 0.55 mL of D2O. The dashed lines are guides to the eye. (f) Comparison of the 1H NMR spectra of original aPEG2k and aPEG2k recovered from the hydrolysis of aPEG2k-1.2TREN.
We also investigated the influence of the water content on the hydrolysis of aPEG2k-Btl by mixing 20 mg of aPEG2k-Btl and different volumes of D2O (200, 300, 500, 700 μL) at 25 °C (Figure S22a). The extent of hydrolysis was monitored by analyzing the 1H NMR signals (Figures S22b–f). As the amount of D2O was increased from 200 to 700 μL, the extent of hydrolysis for aPEG2k-Btl increased from 17 % to 36 % after 120 min, and from 76 % to 88 % after 9 h (Figure S22f). The results suggest that a large excess of D2O is required besides high temperature to drive the hydrolysis process in VU-based vitrimers containing hydrophilic components.
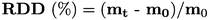 (1)
(1)A comparison of the RDD determined at different temperatures (Figure 3e) shows that, also in this case, higher temperatures result in faster hydrolysis, mirroring the trend observed for aPEG2k-Btl. For instance, the RDD is 24 % after 42 minutes at 20 °C, while it increases to 57 % after 15 min at 80 °C (Figure 3e).
We further investigated how the composition of aPEGx-yTREN influences the water-promoted depolymerization. The first set of experiments was conducted with the aPEGx-1.2TREN series at room temperature (page S27). All of the aPEGx-1.2TREN/H2O mixtures (1 : 15 wt : wt) were initially heterogeneous, and eventually turned into clear solutions (Figure S28). We also investigated the influence of the TREN feed on the depolymerization of aPEG2k-yTREN, and the comparison shows that the time to full dissolution increases with the TREN content. The data suggest that the kinetics of the water-assisted depolymerization of the aPEGx-yTREN can be engineered to occur within a broad time range (Figure S29), although we emphasize that the experiments reported here ultimately probe the dissolution and not the complete depolymerization of the VU networks.
We next pursued the recovery of aPEGx (x<35 k) or aPTHF2k (Figures S26–28). Our strategy to separate aPEGx (x≤35k) and TREN was inspired by previous work from the Helms lab10a and leveraged the difference in the chemical properties of these two components (page S30). In order to remove TREN, the clear solutions produced by depolymerization for 24 h at room temperature in water (conditions discussed above) were treated with a strongly acidic ion exchange resin, which was subsequently filtered off. Evaporation of the solvent (H2O) from the filtrate afforded aPEGx in a yield of 97 %–99 % (page S27). The low amounts of TREN used in the preparation of the VU vitrimers (<150 mg; Table S1) made the quantitative recovery of this component particularly challenging. Thus, to show the feasibility of the applied procedure, we carried out a model experiment in which the acidic ion exchange resin was impregnated with 0.5 g of TREN and further treated with an excess of trimethylamine. Filtration of the thus treated resins afforded the recovery of TREN in a yield of 88 % (Figure S27). The purity of the recovered aPEGx and TREN was assessed by 1H NMR spectroscopy, which confirmed the chemical structures and suggested the absence of (detectable) impurities (Figure 3f; S30–35).
As discussed above, aPTHF2k-1.2TREN remains mechanically stable in neutral or basic water, even after being immersed for 3 days, and therefore this hydrophobic VU vitrimer requires different depolymerization conditions than aPEGx-yTREN. Gratifyingly, aPTHF2k-1.2TREN could be degraded by treatment with a 1 M HCl aqueous solution at room temperature. The degradation process occurred within 3 days, after which the initially clear liquid phase had turned into an off-white slurry. Extraction with CHCl3, neutralization of the organic phase through washing with water, and removal of the solvent allowed the recovery of aPTHF2k in high yield (95 %, page S28) and in high purity (Figure S26b).
We explored the recyclability of a (nano)filler-reinforced composite material that was prepared by combining multi-walled carbon nanotubes (MCNs) with aPEG10k-1.2TREN (aPEG10k-1.2TREN/MCNs) (synthesis on page S38). Immersing aPEG10k-1.2TREN/MCNs in H2O for 24 h afforded a suspension, from which the MCNs could be filtered off, leaving a clear solution containing aPEG10k and TREN (Figure S36). Further treatment with the acidic ion exchange resin permitted the almost quantitative recovery (96 % of the initial mass) of aPEG10k (Figure S36). Scanning electron microscopy (SEM) imaging and Raman spectroscopy provided qualitative indications of the unperturbed morphology of the recovered MCNs (Figure S37).
Finally, we tested the possibility to apply the depolymerization/separation sequence to VU networks in complex mixtures that simulated a mixed waste stream (Figure S38). The experiment was conducted by mixing aPEG10k-1.2TREN with household waste (polyethylene, polypropylene, aluminum foil, tree bark, and leaves) or commodity polymer waste (polyvinyl chloride, polyethylene terephthalate, polycarbonate, and polystyrene). The mixtures were treated with water (1500 weight %) for 24 h at room temperature, and the solid components were removed via filtration. After treatment of the obtained solutions with the acidic ion exchange resin, filtration, and water evaporation, aPEG10k was recovered with a negligible loss (ca. 97 % of material recovered) and in high purity, as confirmed by its 1H NMR spectrum (Figures S38–39).
Thermal Reprocessing and Water-Assisted Recycling of the Vinylogous Urethane Vitrimers
As previously demonstrated by Du Prez et al., VU linkages can undergo transamination processes in the presence of auxiliary amines at sufficiently high temperature (60–150 °C) (Figure 4a, notice the exchange between the blue and green amines).19b, 20a, 24 Such transamination offers two paths to recycle VU vitrimers. The first one involves the thermal reprocessing at 120–150 °C,10b, 10c while the second one relies on the depolymerization by treatment with monofunctional amines at 60–120 °C, followed by repolymerization with a fresh amine cross-linker.24a, 24c-24f In addition to these established methods, we hypothesized that the water-mediated reversible (de)polymerization of VU-based polymer materials reported here could serve as a complementary reprocessing strategy. Thus, we attempted the reprocessing of the aPEG2k-yTREN series by either activating the dynamic transamination process at 150 °C (Figure 4a, left), or by exploring the water-dependent depolymerization-polymerization sequence (Figure 4a, right).
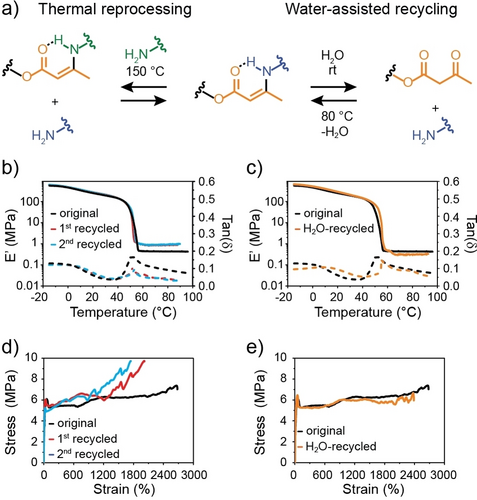
(a) The transamination reaction between an amine and a vinylogous urethane during thermal reprocessing (left) and the reversible, water-assisted depolymerization of a vinylogous urethane into the parent acetoacetyl- and amine-containing compounds. (b) DMA traces and (c) stress-strain curves of an as-prepared, solution-cast aPEG2k-1.2TREN film, and recycled films produced by compression-molding at 150 °C (see text for details). (d) DMA traces and (e) stress-strain curves of an as-prepared, solution-cast aPEG2k-1.2TREN film and a film produced by water-assisted closed-loop recycling (see text for details).
The dynamic character of the transamination of the aPEGx-yTREN systems was initially investigated in solution with model compound aPEG2k-Btl and benzylamine (page S44). The two compounds were mixed in deuterated DMSO (DMSO-d6) in a 1 : 6 aPEG2k-Btl:benzylamine molar ratio, and the resulting mixtures were heated at 80 °C, 100 °C, and 120 °C (Figure S40). The progress of the exchange reaction was monitored by integrating the 1H NMR signals of the VU linkages comprising the two amine substituents (Figure S40), which also allowed us to establish the kinetic profiles at each reaction temperature (Figure S41; Table S6), and determine the Ea of the transamination process, which is very low (35 kJ mol−1) (Figure S42).
We then probed if the transamination process would allow the aPEG2k-yTREN films to flow by performing stress relaxation experiments at different temperatures above the Tm of the PEG domains (Figure S43). No such experiments could be carried out for aPEG2k-1.0TREN, which features a low cross-link density and flows upon melting (Figures 1d, S15). By contrast, the stress-relaxation curves of aPEG2k-1.2TREN and aPEG2k-1.5TREN reveal relaxations processes that we analyzed using the Maxwell model for stress-relaxation (Figures S43b, S43d, page S46). A comparison of the stress relaxation curves shows that, under the same conditions, stresses are dissipated more rapidly in aPEG2k-1.5TREN than in aPEG2k-1.2TREN. For instance, at 130 °C, the relaxation time (τ), at which the stress is dissipated to 1/e of its initial value, is 4.5 min for aPEG2k-1.5TREN and 50 min for aPEG2k-1.2TREN (Figures S43a, S43c). We attribute such difference in stress relaxation efficiency to a larger amount of free amines available in the aPEG2k-1.5TREN network, which is tantamount to stating that a higher loading of free amines provides more efficient transamination processes.10c, 25 Linear fits of ln(τ) versus 1/T afforded Ea values of 81.4 kJ mol−1 for aPEG2k-1.2TREN and 127.1 kJ mol−1 for aPEG2k-1.5TREN for the topological rearrangement, which comprises the transamination and other processes, of the polymer networks (Table S7). Moreover, by fitting the data to a Maxwell equation and assuming a Poisson's ratio of 0.5,26 we could identify the theoretical topology-freezing temperature (Tv) (calculations on page S47, Table S7), above which the exchange reaction in the polymer material becomes active. The Tv of aPEG2k-1.2TREN is 66 °C, while a value of 40 °C was determined for aPEG2k-1.5TREN.
We next investigated the possibility to thermally reprocess aPEG2k-1.2TREN. A film of aPEG2k-1.2TREN was cut into small pieces, which were then compression-molded at 150 °C and a pressure of 10 MPa for 30 min. This treatment afforded a homogeneous film (Figure S44). The process was repeated and the mechanical properties of the original aPEG2k-1.2TREN film and the samples made in the first and second recycling step were compared by DMA (Figure 4b) and tensile testing (Figure 4c). Gratifyingly, the mechanical properties of the three samples are almost indistinguishable. The DMA traces of the thermally recycled aPEG2k-1.2TREN show a slight increase of E′ in the rubbery regime (0.9 MPa for the recycled vs 0.5 MPa for the original aPEG2k-1.2TREN), whereas the tensile tests reveal an increase in the stress at break (9.7 MPa for the recycled vs 7.0 MPa for the original aPEG2k-1.2TREN) and a decrease in the strain at break (2020 % for the thermally recycled vs 2630 % for the original aPEG2k-1.2TREN). These differences might be related to some unreacted amines and acetoacetate moieties in the as-prepared material, which was produced by solution casting, which were converted into VU bonds via thermal reprocessing. This seems to be supported by the lack of significant differences observed in the DMA traces and stress-strain curves of the 1st and 2nd thermally recycled films (Figures 4b–c), which suggests similar network architectures and density of cross-links. The hydrolysis of the VU linkages upon reprocessing could be ruled out, as this would have caused a decrease in E′ due to lowering of the density of cross-links.
Finally, we conducted similar study experiments and compared the mechanical properties of the original aPEG2k-1.2TREN to those of an aPEG2k-1.2TREN that was produced from depolymerized matter. Briefly, we treated aPEG2k-1.2TREN with a 5-fold excess of deionized H2O at 25 °C, which caused hydrolysis of the VU linkages and the solubilization of aPEG2k and TREN (Figure S28). The solution thus produced was subsequently cast into a Teflon mold, heated to 80 °C overnight, and finally dried in vacuum at 80 °C for 12 h. The DMA and stress-strain profiles obtained by measuring the original and re-synthesized (H2O-recycled) polymer show that the two materials display practically identical mechanical properties (Figures 4d–e), demonstrating the effectiveness of water-assisted recycling of aPEG2k-1.2TREN.
Conclusion
In conclusion, we have presented a case study of the closed-loop recycling behavior of vinylogous urethane vitrimers based on bis-acetoacetate terminated polyethylene glycol and tris(2-aminoethyl) amine. In stark contrast to polymers comprising bonds derived from irreversible bond-forming reactions, polymers featuring highly dynamic covalent bonds are easier to recycle, and further allow the recovery of the original monomer in high purity and efficiency with limited costs. Indeed, the vinylogous urethane vitrimers here presented allow the almost quantitative recovery of the bis-acetoacetate terminated polyethylene glycol (ca. 98 %). The depolymerization rate can be easily adjusted by judicious choice of the reaction temperature, the amount of water, the molecular weight of the bis-acetoacetate terminated polyethylene glycol, and the stoichiometric ratio of the two starting materials. These two parameters also allow to fine tune the mechanical properties of the final materials, with formulations that proved to be soft, tough, and/or brittle. The here reported vinylogous urethanes can be reprocessed multiple times by thermal processing, and by a circular depolymerization-repolymerization scheme assisted by water. While the former has already been demonstrated, the latter is a concept that, to our best knowledge, was missing in the rapidly growing literature of vinylogous urethane vitrimers.
Supporting Information
The authors have cited additional references within the Supporting Information.27, 28
Acknowledgments
The National Natural Science Foundation of China (52073171, 51873104) is acknowledged for financial support. Y.W.M., C.W., and J.A.B. are thankful to the Adolphe Merkle Foundation for generous financial support. All authors thank the China Scholarship Council for supporting Y.W.M.’s stay at the Adolphe Merkle Institute as a visiting scholar for one year (202006230307). Open Access funding provided by Université de Fribourg.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.