Supramolecular Enhancement of Electrochemical Nitrate Reduction Catalyzed by Cobalt Porphyrin Organic Cages for Ammonia Electrosynthesis in Water**
Dr. Lun An
Department of Chemistry, University of California, Berkeley, Berkeley, CA, 94720-1460 USA
Chemical Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA, 94720-1460 USA
Search for more papers by this authorDr. Mina R. Narouz
Department of Chemistry, University of California, Berkeley, Berkeley, CA, 94720-1460 USA
Chemical Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA, 94720-1460 USA
Search for more papers by this authorDr. Peter T. Smith
Department of Chemistry, University of California, Berkeley, Berkeley, CA, 94720-1460 USA
Chemical Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA, 94720-1460 USA
Search for more papers by this authorPatricia De La Torre
Department of Chemistry, University of California, Berkeley, Berkeley, CA, 94720-1460 USA
Chemical Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA, 94720-1460 USA
Search for more papers by this authorCorresponding Author
Prof. Christopher J. Chang
Department of Chemistry, University of California, Berkeley, Berkeley, CA, 94720-1460 USA
Chemical Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA, 94720-1460 USA
Department of Molecular and Cell Biology, University of California, Berkeley, Berkeley, CA, 94720-1460 USA
Search for more papers by this authorDr. Lun An
Department of Chemistry, University of California, Berkeley, Berkeley, CA, 94720-1460 USA
Chemical Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA, 94720-1460 USA
Search for more papers by this authorDr. Mina R. Narouz
Department of Chemistry, University of California, Berkeley, Berkeley, CA, 94720-1460 USA
Chemical Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA, 94720-1460 USA
Search for more papers by this authorDr. Peter T. Smith
Department of Chemistry, University of California, Berkeley, Berkeley, CA, 94720-1460 USA
Chemical Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA, 94720-1460 USA
Search for more papers by this authorPatricia De La Torre
Department of Chemistry, University of California, Berkeley, Berkeley, CA, 94720-1460 USA
Chemical Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA, 94720-1460 USA
Search for more papers by this authorCorresponding Author
Prof. Christopher J. Chang
Department of Chemistry, University of California, Berkeley, Berkeley, CA, 94720-1460 USA
Chemical Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA, 94720-1460 USA
Department of Molecular and Cell Biology, University of California, Berkeley, Berkeley, CA, 94720-1460 USA
Search for more papers by this authorA previous version of this manuscript has been deposited on a preprint server (https://doi.org/10.26434/chemrxiv-2022-09q8t-v2).
Graphical Abstract
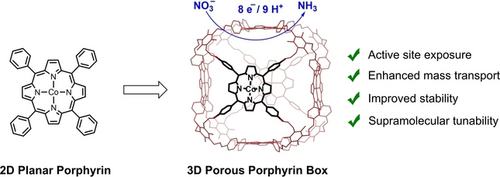
We present a supramolecular strategy for electrochemical nitrate reduction by augmenting porosity and electrochemically active sites as well as facilitating catalyst-substrate interactions. The resulting 3D architecture enables selective ammonia production in water with greater than 90 % Faradaic efficiency and turnover numbers exceeding 200,000, representing a 15-fold increase in activity and higher catalytic stability over its 2D counterpart.
Abstract
The electrochemical nitrate (NO3−) reduction reaction (NO3RR) to ammonia (NH3) represents a sustainable approach for denitrification to balance global nitrogen cycles and an alternative to traditional thermal Haber-Bosch processes. Here, we present a supramolecular strategy for promoting NH3 production in water from NO3RR by integrating two-dimensional (2D) molecular cobalt porphyrin (CoTPP) units into a three-dimensional (3D) porous organic cage architecture. The porphyrin box CoPB-C8 enhances electrochemical active site exposure, facilitates substrate–catalyst interactions, and improves catalyst stability, leading to turnover numbers and frequencies for NH3 production exceeding 200,000 and 56 s−1, respectively. These values represent a 15-fold increase in NO3RR activity and 200-mV improvement in overpotential for the 3D CoPB-C8 box structure compared to its 2D CoTPP counterpart. Synthetic tuning of peripheral alkyl substituents highlights the importance of supramolecular porosity and cavity size on electrochemical NO3RR activity. These findings establish the incorporation of 2D molecular units into 3D confined space microenvironments as an effective supramolecular design strategy for enhancing electrocatalysis.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202305719-sup-0001-misc_information.pdf2.1 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1M. M. M. Kuypers, H. K. Marchant, B. Kartal, Nat. Rev. Microbiol. 2018, 16, 263–276.
- 2X. Zhang, B. B. Ward, D. M. Sigman, Chem. Rev. 2020, 120, 5308–5351.
- 3
- 3aJ. N. Galloway, A. Bleeker, J. W. Erisman, Annu. Rev. Environ. Res. 2021, 46, 255–288;
- 3bJ. N. Galloway, A. R. Townsend, J. W. Erisman, M. Bekunda, Z. Cai, J. R. Freney, L. A. Martinelli, S. P. Seitzinger, M. A. Sutton, Science 2008, 320, 889–892.
- 4Bijay-Singh, E. Craswell, SN Appl. Sci. 2021, 3, 518.
- 5N. Lehnert, H. T. Dong, J. B. Harland, A. P. Hunt, C. J. White, Nat. Chem. Rev. 2018, 2, 278–289.
- 6M. H. Ward, R. R. Jones, J. D. Brender, T. M. De Kok, P. J. Weyer, B. T. Nolan, C. M. Villanueva, S. G. Van Breda, Int. J. Environ. Res. Public Health 2018, 15, 1557.
- 7C. J. Werth, C. X. Yan, J. P. Troutman, ACS ES&T Eng. 2021, 1, 6–20.
- 8R. C. Scholes, M. A. Vega, J. O. Sharp, D. L. Sedlak, Environ. Sci.: Water Res. Technol. 2021, 7, 650–661.
- 9F. F. Rivera, E. P. Rivero, F. Castaneda-Zaldivar, Ind. Eng. Chem. Res. 2021, 60, 5014–5023.
- 10N. Barrabés, J. Sá, Appl. Catal. B 2011, 104, 1–5.
- 11
- 11aV. Rosca, M. Duca, M. T. de Groot, M. T. M. Koper, Chem. Rev. 2009, 109, 2209–2244;
- 11bM. Duca, M. T. M. Koper, Energy Environ. Sci. 2012, 5, 9726–9742;
- 11cY. Zeng, C. Priest, G. Wang, G. Wu, Small Methods 2020, 4, 2000672;
- 11dB. Min, Q. Gao, Z. Yan, X. Han, K. Hosmer, A. Campbell, H. Zhu, Ind. Eng. Chem. Res. 2021, 60, 14635–14650.
- 12
- 12aP. H. van Langevelde, I. Katsounaros, M. T. M. Koper, Joule 2021, 5, 290–294;
- 12bG. Qing, R. Ghazfar, S. T. Jackowski, F. Habibzadeh, M. M. Ashtiani, C.-P. Chen, M. R. Smith, T. W. Hamann, Chem. Rev. 2020, 120, 5437–5516;
- 12cA. Valera-Medina, F. Amer-Hatem, A. K. Azad, I. C. Dedoussi, M. de Joannon, R. X. Fernandes, P. Glarborg, H. Hashemi, X. He, S. Mashruk, J. McGowan, C. Mounaim-Rouselle, A. Ortiz-Prado, A. Ortiz-Valera, I. Rossetti, B. Shu, M. Yehia, H. Xiao, M. Costa, Energy Fuels 2021, 35, 6964–7029;
- 12dD. Hao, Y. Liu, S. Gao, H. Arandiyan, X. Bai, Q. Kong, W. Wei, P. K. Shen, B.-J. Ni, Mater. Today 2021, 46, 212–233.
- 13
- 13aI. Taniguchi, N. Nakashima, K. Matsushita, K. Yasukouchi, J. Electroanal. Chem. 1987, 224, 199–209;
- 13bN. Chebotareva, T. Nyokong, J. Appl. Electrochem. 1997, 27, 975–981;
- 13cJ. Shen, Y. Y. Birdja, M. T. M. Koper, Langmuir 2015, 31, 8495–8501;
- 13dS. Xu, D. C. Ashley, H. Y. Kwon, G. R. Ware, C. H. Chen, Y. Losovyj, X. F. Gao, E. Jakubikova, J. M. Smith, Chem. Sci. 2018, 9, 4950–4958;
- 13eS. E. Braley, D. C. Ashley, E. Jakubikova, J. M. Smith, Chem. Commun. 2020, 56, 603–606;
- 13fS. Partovi, Z. Q. Xiong, K. M. Kulesa, J. M. Smith, Inorg. Chem. 2022, 61, 9034–9039.
- 14
- 14aJ. Li, G. M. Zhan, J. H. Yang, F. J. Quan, C. L. Mao, Y. Liu, B. Wang, F. C. Lei, L. J. Li, A. W. M. Chan, L. P. Xu, Y. B. Shi, Y. Du, W. C. Hao, P. K. Wong, J. F. Wang, S. X. Dou, L. Z. Zhang, J. C. Yu, J. Am. Chem. Soc. 2020, 142, 7036–7046;
- 14bG. F. Chen, Y. F. Yuan, H. F. Jiang, S. Y. Ren, L. X. Ding, L. Ma, T. P. Wu, J. Lu, H. H. Wang, Nat. Energy 2020, 5, 605–613;
- 14cZ. Y. Wu, M. Karamad, X. Yong, Q. Huang, D. A. Cullen, P. Zhu, C. Xia, Q. Xiao, M. Shakouri, F. Y. Chen, J. Y. T. Kim, Y. Xia, K. Heck, Y. Hu, M. S. Wong, Q. Li, I. Gates, S. Siahrostami, H. Wang, Nat. Commun. 2021, 12, 2870;
- 14dZ. Gao, Y. L. Lai, Y. Tao, L. H. Xiao, L. X. Zhang, F. Luo, ACS Cent. Sci. 2021, 7, 1066–1072;
- 14eQ. Gao, B. Yao, H. S. Pillai, W. Zang, X. Han, Y. Liu, S.-W. Yu, Z. Yan, B. Min, S. Zhang, H. Zhou, L. Ma, H. Xin, Q. He, H. Zhu, Nat. Synth. 2023, 2, 624–634;
- 14fY. Lv, S. W. Ke, Y. M. Gu, B. L. Tian, L. Y. Tang, P. Ran, Y. Zhao, J. Ma, J. L. Zuo, M. N. Ding, Angew. Chem. Int. Ed. 2023, 62, e202305246.
- 15
- 15aT. Tozawa, J. T. A. Jones, S. I. Swamy, S. Jiang, D. J. Adams, S. Shakespeare, R. Clowes, D. Bradshaw, T. Hasell, S. Y. Chong, C. Tang, S. Thompson, J. Parker, A. Trewin, J. Bacsa, A. M. Z. Slawin, A. Steiner, A. I. Cooper, Nat. Mater. 2009, 8, 973–978;
- 15bC. S. Diercks, O. M. Yaghi, Science 2017, 355, eaal1585.
- 16
- 16aS. Banerjee, R. I. Anayah, C. S. Gerke, V. S. Thoi, ACS Cent. Sci. 2020, 6, 1671–1684;
- 16bP. T. Smith, E. M. Nichols, Z. Cao, C. J. Chang, Acc. Chem. Res. 2020, 53, 575–587;
- 16cA. H. Proppe, Y. G. C. Li, A. Aspuru-Guzik, C. P. Berlinguette, C. J. Chang, R. Cogdell, A. G. Doyle, J. Flick, N. M. Gabor, R. van Grondelle, S. Hammes-Schiffer, S. A. Jaffer, S. O. Kelley, M. Leclerc, K. Leo, T. E. Mallouk, P. Narang, G. S. Schlau-Cohen, G. D. Scholes, A. Vojvodic, V. W. W. Yam, J. Y. Yang, E. H. Sargent, Nat. Rev. Mater. 2020, 5, 828–846.
- 17
- 17aP. T. Smith, Y. Kim, B. P. Benke, K. Kim, C. J. Chang, Angew. Chem. Int. Ed. 2020, 59, 4902–4907;
- 17bA. N. Oldacre, A. E. Friedman, T. R. Cook, J. Am. Chem. Soc. 2017, 139, 1424–1427.
- 18
- 18aP. T. Smith, B. P. Benke, Z. Cao, Y. Kim, E. M. Nichols, K. Kim, C. J. Chang, Angew. Chem. Int. Ed. 2018, 57, 9684–9688;
- 18bY. Hu, S. Huang, L. J. Wayment, J. Wu, Q. Xu, T. Chang, Y.-P. Chen, X. Li, B. Andi, H. Chen, Y. Jin, H. Zhu, M. Du, S. Lu, W. Zhang, Cell Rep. Phys. Sci. 2023, 4, 101285.
- 19P. T. Smith, B. P. Benke, L. An, Y. Kim, K. Kim, C. J. Chang, ChemElectroChem 2021, 8, 1653–1657.
- 20
- 20aT. A. Barendt, A. Docker, I. Marques, V. Felix, P. D. Beer, Angew. Chem. Int. Ed. 2016, 55, 11069–11076;
- 20bJ. C. Lauer, A. S. Bhat, C. Barwig, N. Fritz, T. Kirschbaum, F. Rominger, M. Mastalerz, Chem. Eur. J. 2022, 28, e202201527;
- 20cM. J. Langton, P. D. Beer, Chem. Commun. 2014, 50, 8124–8127.
- 21X. Wu, E. N. W. Howe, P. A. Gale, Acc. Chem. Res. 2018, 51, 1870–1879.
- 22R. D. Mukhopadhyay, Y. Kim, J. Koo, K. Kim, Acc. Chem. Res. 2018, 51, 2730–2738.
- 23B. P. Benke, P. Aich, Y. Kim, K. L. Kim, M. R. Rohman, S. Hong, I. C. Hwang, E. H. Lee, J. H. Roh, K. Kim, J. Am. Chem. Soc. 2017, 139, 7432–7435.
- 24S. Hong, M. R. Rohman, J. Jia, Y. Kim, D. Moon, Y. Kim, Y. H. Ko, E. Lee, K. Kim, Angew. Chem. Int. Ed. 2015, 54, 13241–13244.
- 25X. M. Hu, M. H. Ronne, S. U. Pedersen, T. Skrydstrup, K. Daasbjerg, Angew. Chem. Int. Ed. 2017, 56, 6468–6472.
- 26S. H. Cheng, Y. O. Su, Inorg. Chem. 1994, 33, 5847–5854.
- 27M. Wang, K. Torbensen, D. Salvatore, S. Ren, D. Joulié, F. Dumoulin, D. Mendoza, B. Lassalle-Kaiser, U. Işci, C. P. Berlinguette, M. Robert, Nat. Commun. 2019, 10, 3602.
- 28L. An, P. De La Torre, P. T. Smith, M. R. Narouz, C. J. Chang, Angew. Chem. Int. Ed. 2023, 62, e202209396.
- 29
- 29aL. Adriaenssens, C. Estarellas, A. V. Jentzsch, M. M. Belmonte, S. Matile, P. Ballester, J. Am. Chem. Soc. 2013, 135, 8324–8330;
- 29bD. X. Wang, M. X. Wang, Acc. Chem. Res. 2020, 53, 1364–1380;
- 29cM. M. Watt, L. N. Zakharov, M. M. Haley, D. W. Johnson, Angew. Chem. Int. Ed. 2013, 52, 10275–10280.
- 30A. Ali, W. Akram, H.-Y. Liu, Molecules 2019, 24, 78.
- 31
- 31aS. Xu, H. Y. Kwon, D. C. Ashley, C. H. Chen, E. Jakubikova, J. M. Smith, Inorg. Chem. 2019, 58, 9443–9451;
- 31bY. X. Guo, J. R. Stroka, B. Kandemir, C. E. Dickerson, K. L. Bren, J. Am. Chem. Soc. 2018, 140, 16888–16892;
- 31cJ. R. Stroka, B. Kandemir, E. M. Matson, K. L. Bren, ACS Catal. 2020, 10, 13968–13972.
- 32S. E. Braley, J. Z. Xie, Y. Losovyj, J. M. Smith, J. Am. Chem. Soc. 2021, 143, 7203–7208.
- 33X. Zhang, Y. T. Wang, Y. B. Wang, Y. M. Guo, X. Y. Xie, Y. F. Yu, B. Zhang, Chem. Commun. 2022, 58, 2777–2787.
- 34E. Berardo, R. L. Greenaway, L. Turcani, B. M. Alston, M. J. Bennison, M. Miklitz, R. Clowes, M. E. Briggs, A. I. Cooper, K. E. Jelfs, Nanoscale 2018, 10, 22381–22388.
- 35M. Miklitz, K. E. Jelfs, J. Chem. Inf. Model. 2018, 58, 2387–2391.
- 36Y. Marcus, Chem. Rev. 1988, 88, 1475–1498.