Compartmentalized Polyampholyte Microgels by Depletion Flocculation and Coacervation of Nanogels in Emulsion Droplets
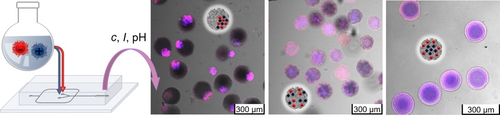
Graphical Abstract
Novel step-by-step approach for the synthesis of polyampholyte nanogel-in-microgel colloids (NiM−C) was developed by incorporating nanogels into microgels via droplet-based microfluidics yielding different internal morphologies including phase-separated coacervates, statistically distributed coacervates and core–shell like arrangements by variation of synthesis parameters such as nanogel concentration, ionic strength and pH value.
Abstract
In pH-responsive drug carriers, the distribution of charges has been proven to affect delivery efficiency but is difficult to control and verify. Herein, we fabricate polyampholyte nanogel-in-microgel colloids (NiM−C) and show that the arrangement of the nanogels (NG) can easily be manipulated by adapting synthesis conditions. Positively and negatively charged pH-responsive NG are synthesized by precipitation polymerization and labelled with different fluorescent dyes. The obtained NG are integrated into microgel (MG) networks by subsequent inverse emulsion polymerization in droplet-based microfluidics. By confocal laser scanning microscopy (CLSM), we verify that depending on NG concentration, pH value and ionic strength, NiM−C with different NG arrangements are obtained, including Janus-like phase-separation of NG, statistical distribution of NG, and core–shell arrangements. Our approach is a major step towards uptake and release of oppositely charged (drug) molecules.
Introduction
A fascinating feature of living systems is their ability to use molecular structures exhibiting functionality on the nanometer scale and translate them into more complex entities via self-organization and compartmentalization in aqueous media. In nature, information is stored by multiple sequential levels of organization, and even the origins of life seem to be related to the compartmentalization of molecular structures and energy carriers.1 Therefore, understanding these principles will help to develop new bio-inspired sustainable materials for catalysis, robotics and medicine.2-4 By designing systems with spatially distinct components, multiple functionalities can be combined without mutual deactivation and loss of effect.5 This may be especially interesting in the conception of new drug carriers. For efficient use in medicinal therapy, drug molecules must be shielded from harsh environmental conditions such as strong pH shifts. The active substances should only be released in the vicinity of the diseased site to reduce undesired side effects and lower the costs.6 Various colloidal structures (e.g. vesicles, liposomes, micelles) have already been considered as carriers for drugs.7, 8, 9 Stimuli-responsive microgels are promising candidates for targeted drug delivery since their internal structure can be compartmentalized and the release of the payload can be stimulated by an environmental trigger.10
Microgels are microscale networks of three-dimensionally crosslinked polymers that are distinct from other (macromolecular) materials as they exhibit dualistic properties of hard nanoparticles and soft macromolecules. Once swollen in a solvent, microgels contain high amounts of the solvent molecules because of their open and porous network structure.11 When the chemical composition of the microgel network is tuned, a sensitivity to a stimulus—such as temperature,12 pH value,13 ionic strength,14 light,15 or ultrasonication16—can be introduced. Such a change in environment triggers the swelling or collapse of the microgel. Prominent examples of responsive microgels are colloidal hydrogels based on poly(N-isopropylacrylamide) (PNIPAAm), which have been extensively studied because of their temperature-responsivity and low cytotoxicity. As the linear PNIPAAm chains have a lower critical solution temperature (LCST), their crosslinked networks undergo a volume-phase transition (VPT) at physiological temperatures >32 °C.12a, 17
In addition to undergoing VPT, PNIPAAm networks can become pH-sensitive and hence, multi-responsive, when weakly acidic or basic comonomers are incorporated.13a, 14a, 18 These ionizable microgels can be used to transport charged guest molecules as they allow for the targeted uptake and release of said molecule by electrostatic interaction.19 Because of an abundance of charged biomolecules in pharmacology,20 pH-responsive microgels are promising candidates for therapeutic drug delivery.
However, larger biomolecules, such as DNA or proteins, assemble into 3D structures that do not fit the size constraints of microgels synthesized by conventional polymerization methods such as precipitation polymerization or inverse miniemulsion polymerization.21 Also, complex diseases necessitate the delivery of multiple drugs that differ in charge or hydrophilicity, or must be released consecutively.22
To overcome these challenges, Peppas et al. synthesized hydrogel films from poly(methacrylic acid-co-N-vinyl-2-pyrrolidone) (P(MAAc-NVP)) containing polycationic 2-(diethylamino)ethyl methacrylate-based nanogels. The dried hydrogel films were mechanically crushed and sieved to yield microgels <75 μm.23 They showed that the anionic microgel network could selectively and timely be degraded by trypsin when an enzyme-labile crosslinker was employed, releasing the nanogels, which in turn induced endocytosis and facilitated the endosomal escape of their cargo.23b A major drawback of this approach is the heterogeneity of the samples in terms of size and shape, which affects experimental reproducibility.
By fabrication of microgels in microfluidic aqueous droplets, their size can be precisely adjusted within a range of 10–1000 μm and both internal and external morphology can be accurately controlled.24 Here, the formation of polyelectrolyte or polyampholyte microgels by incorporation of transiently charged monomer moieties is well established, too, and has been proven to allow for encapsulation of nutrient molecules or model drugs.25 Additionally, Weitz et al. introduced new functionalities into microgels by embedding different materials including quantum dots, magnetic nanoparticles and polymer microparticles into microgel networks. Even after insertion of additives, the temperature-responsivity of the original PNIPAAm network was retained.26 Santos et al. integrated halloysite nanotubes into microgel networks based on hydroxylpropyl methylcellulose acetate succinate. They proved that this system could simultaneously take up two model drugs (atorvastatin and celecoxib), which differ in their physicochemical properties but, when combined, efficiently combat colon cancer. The polymer network was stable in acidic medium but dissolved in a neutral to basic environment, facilitating the quick and targeted release of the co-loaded drugs.27 Similarly, Janus-like microgels were fabricated. For that, cationic temperature-responsive nanogels were mixed with poly(acrylic acid), acrylamide monomer, crosslinker and initiator in aqueous droplets. An increase in temperature and electrostatic interactions between cationic nanogels and anionic polymers then forced phase-separation, which was preserved by immediate polymerization.28 Recently, the group of Isa proposed supracapsules for multi-drug delivery. They employed a microfluidic cross-junction to form oil droplets containing dextran-based nanogels, which spontaneously assemble into supraparticles when evaporating the solvent. The group showed that the van-der-Waals forces responsible for holding the nano-compartments together can be broken by a high-energy input in form of ultrasonication, causing the supraparticles to separate into singular entities again.29 While these compartmentalized supracapsules allow for the transport and release of multiple drug payloads, it remains challenging to integrate cargoes exhibiting opposing physicochemical properties or charges.
Previous work focused on embedding charged or hydrophobic species into anionic hydrophilic networks.23, 27, 28 However, integration of two oppositely charged polyelectrolyte nanogel species potentially opens up ways for orthogonal drug delivery. In this work, we demonstrate for the first time the synthesis of compartmentalized polyampholyte microgels (MG) by depletion flocculation and coacervation of polyelectrolyte nanogels (NG) in emulsion droplets. For the sake of clarity and readability, we herein use the term “nanogels” for polymer gels prepared by precipitation polymerization even though their diameter (approx. 400–500 nm in HPLC-grade water) exceeds the size range for nanogels as defined by IUPAC.30 The different designation should facilitate differentiation between the polymer networks since the size of microgels produced by microfluidics is several orders of magnitude larger. In a droplet-based microfluidic device, dispersions of oppositely charged NG, synthesized by conventional precipitation polymerization, are integrated into aqueous emulsion droplets followed by polymerization and crosslinking of N-isopropylacrylamide. The macro-responsivity of the nanogel-in-microgel colloids (NiM−C) is examined regarding pH value, ionic strength and temperature, verifying that the NiM−C react similarly to pure PNIPAAm MG. By labelling the NG with fluorescent tags, we analyze the arrangement of the NG within the NiM−C by use of confocal laser scanning microscopy (CLSM). We found that Janus-like structures form as the NG coacervate and accumulate on one side of the MG network. We make a plausible case that driving factors for this coacervation mechanism are the electrostatic interactions between the two NG species and depletion forces arising from the formation of polymer chains. We show that due to the responsive character of the charged NG, their arrangement within the MG can be tuned by adjusting synthesis conditions, i.e. NG concentration, ionic strength and pH value.
Results and Discussion
Our aim was to develop a robust synthesis method to obtain micron-sized gels that, under physiological conditions, contain positively and negatively charged compartments for efficient drug encapsulation. We selected PNIPAAm-based networks crosslinked with N,N′-methylenebis(acrylamide) (BIS) as model system, since NIPAAm is one of the most widely used monomers and exhibits temperature-induced VPT close to human body temperature.17a, 31 Weakly acidic methacrylic acid (MAAc, pKA≈5.032) and weakly basic N-(3-aminopropyl)methacrylamide (APMH, pKA≈10.033) were introduced as pH-responsive co-monomers, because they are water-soluble and oppositely charged at neutral pH value. Polyelectrolyte NG with a hydrodynamic diameter of 400–500 nm and a co-monomer content of 10 mol% were synthesized by precipitation polymerization (Supporting Information, Table S1). As described in literature,13a synthesis conditions had to be carefully adjusted to provide the desired incorporation of the respective ionizable co-monomer. For the synthesis of NG containing APMH (pNG), the pH value was adjusted to a basic value to ensure deprotonation of the co-monomer. As has been shown for the free radical polymerization of ionic monomers in aqueous solution, the reaction kinetic is negatively affected by electrostatic interactions between the growing polymer and counterions, other polymer backbones or the radical species.34 The batch precipitation polymerization was initiated with the cationic azo initiator 2,2′-azobis-(2-methyl-propionamidine) (AMPA, V-50). In the synthesis of NG containing MAAc (nNG), the faster consumption of MAAc compared to NIPAAm was accounted for by adding the co-monomer belatedly to the acidic reaction mixture. 2,2′-azobis[N-(2-carboxyethyl)-2-methylpropionamidine] tetra-hydrate (ACMA, VA-057) was used as initiator and anionic sodium dodecyl sulfate (SDS) was added as surfactant to obtain nNG sizes comparable to the pNG. To efficiently distinguish the NG species visually and in CLSM, the nNG were labelled by adding acryloxyethyl thiocarbamoyl Rhodamine B (Rhod B Ac) to the polymerization mixture. The pNG were tagged with cyanine 5 N-hydroxysuccinimide ester (Cy5 NHS) in a post-modification reaction by conjugating the NHS ester to primary amines in the NG network. After purification by automated tangential-flow diafiltration (TFF),35 the NG were investigated regarding size and temperature-, pH-, and ionic strength responsivity by scanning-transmission electron microscopy (STEM, Figure S2), dynamic light scattering (DLS, Figure S3) and electrophoretic light scattering (ELS, Figure S4). The results show that nNG have a hydrodynamic diameter of around 400 nm in HPLC-grade water at room temperature whereas pNG are about 470 nm in size under identical conditions. They both exhibit a polydispersity index of <0.09, indicating a narrow size distribution. Both types of NG exhibit the expected thermo-, pH-, and ionic strength responsive behavior (Figure S3). ELS measurements confirmed a negative effective surface charge of the nNG at pH values above pKA, and a positive effective surface charge of the pNG at pH values below pKA (Figure S4). We quantified the co-monomer content of the NG by subjecting samples to proton nuclear magnetic resonance (1H NMR ), and infrared spectroscopy (IR) (Figure S5–S8). The quantitative APMH content was determined by 1H NMR to be 8.4 mol% (Figure S7). As a method for assessing the MAAc content, 1H NMR is unsuitable, because the signals of NIPAAm and MAAc moieties overlap. Quantification was established using the corresponding carboxyl signal in IR. The content of MAAc was 8.0 mol% after labelling (Figure S5, S8).
The labelled NG were incorporated into MG networks via droplet-based microfluidic synthesis, which allows for the fabrication of monodisperse colloidal hydrogels that can easily be adjusted by varying the flow rates and contents of the organic and aqueous phases.36 Again, NIPAAm and BIS were used for the gel network. For stabilization of the water-in-oil droplets, the uncharged surfactant FluoSurf was employed in the continuous fluorinated oil phase, thus avoiding additional charges that potentially influence the reaction. The polymerization was started with the UV-initiator lithium-phenyl-2,4,6-trimethylbenzoylphosphinate (LAP), which has good water-solubility and fast UV reactivity (365 nm). LAP is known to suppress premature gelation and, in this respect, allows for spatial and temporal control of the photopolymerization.37 We decided on a UV-initiator instead of a conventional azoinitiator to avoid heating the dispersion, as this would induce precipitation of the NG.
For the microfluidic reactions, NIPAAm, BIS and LAP were dissolved in the respective NG dispersion (10 mg/mL) to ensure homogeneous distribution of the NG. To obtain polyelectrolyte NiM−C, the dispersed aqueous phase included either nNG or pNG, and only one inlet was used (Figure S1). For the fabrication of polyampholyte NiM−C, both types of NG were incorporated by simultaneously using two distinct aqueous dispersions, one for each inlet. At the cross-flow junction (Figure 1 (A)), the shear forces between the two immiscible fluids led to the formation of water-in-oil droplets, which then passed the mixing section (Figure 1 (B)), where laminar flow changed to turbulent flow as a result of the serpent-shaped channel.38 After collecting the droplets (Figure 1 (C)), the emulsion was irradiated with UV light. An irradiation time of 10 s was optimal as polymerization took place but photobleaching effects were kept at a minimum. Differences in MG size between samples can be attributed to minute deviations in monomer weight, or differences in the flow rates resulting from the accuracy of the syringe pumps.
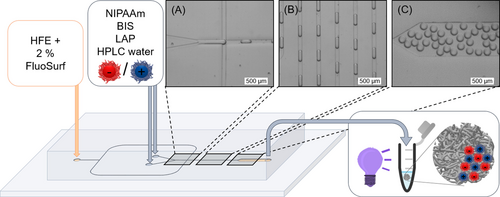
Microfluidic chip layout for the production of polyampholyte NiM−C. FluoSurf in fluorinated oil was used as continuous phase, HPLC-grade water as dispersed phase. For both water phases, reactants are dissolved in a dispersion of one type of polyelectrolyte NG. Water-in-oil droplets are formed at the cross-flow junction (A), then mixed (B), collected at the outlet (C) and irradiated for 10 s with UV light to initiate the polymerization. Scale bars represent 500 μm.
After purification, we studied the NiM−C regarding morphology and localization of the fluorescently labelled NG by CLSM imaging. For both types of polyelectrolyte NiM−C, Figure 2 reveals a statistical distribution of NG where the Rhod B labelled nNG are colored red, and the Cy5 labelled pNG are colored blue. Both NG species are swollen in water at room temperature (Figure S3, (C)) because of the repulsive electrostatic interactions between the moieties carrying the same charge and the ensuing osmotic pressure of the native counterions.39 We hypothesize that these interactions also have a long-range effect and thus, induce the spatially distributed NG arrangement. The formation, growth and crosslinking of PNIPAAm chains does apparently not affect the colloidal stability of polyelectrolyte NG before they become entrapped in the polymer network. Reference experiments with pure PNIPAAm NG show a similarly homogeneous distribution of the NG within the MG network (Figure S9). In neutral NG, which do not contain charged co-monomers, colloidal stability results from dangling chains and a minor electrostatic contribution is caused by integrated initiator-derived groups and Rhod B Ac labelling agent. It is likely that these stabilization mechanisms are already sufficient to induce the statistical distribution of the NG. In contrast to that, the polyampholyte NiM−C that contain both types of NG exhibit a Janus-like structure in which the two NG species form highly phase-separated coacervates that settle to one side within the MG network. As proven by the pink color in the overlay images (Figure 2, Figure 3), resulting from the combination of nNG (red) and pNG (blue), the coacervates consist of both NG species. This observation indicates that the attractive electrostatic interactions between oppositely charged NG are the driving force of coacervation, just as the repulsion between NG of the same charge causes spatial distribution of the NG within the entire MG network. Simply mixing the two polyelectrolyte NG dispersions does not lead to visible or microscopically detectable coacervation (Figure S10, Figure 4). By viscometry, we approximate that the generalized effective volume fraction of the NG in dispersion was below colloidal glass transition (Figure S11).

CLSM images and Schemes of polyelectrolyte NiM−C with nNG ((A)–(D)) and pNG ((E)–(H)), and of polyampholyte NiM−C ((I)–((L)) in HPLC-grade water (pH≈6.5). In the first row from the left ((A), (E) and (I)), fluorescence images (DPSS laser: λex=561 nm, HyD detector) show the location of Rhod B labelled nNG (red). In the second row ((B), (F) and (J)), fluorescence images (HeNe laser: λex=633 nm, HyD detector) indicate the positions of the Cy5 labelled pNG (blue). Overlay images (right row: (D), (H) and (L)) combine both fluorescence and brightfield images (PMT detector:≈300 V) ((C), (G) and (K)). Scale bars represent 300 μm.
The overall responsiveness of the NiM−C to temperature, pH value and ionic strength was tested. Temperature-dependent size measurements show that, above VPTT, the diameters of the NiM−C decrease similar to the deswelling of pure PNIPAAm MG. However, the VPTT determined by sigmoidal Boltzmann fit is increased for the PNIPAAm MG and NiM−C (Figure 3, Figure S12) compared to the VPTT of colloidal PNIPAAm gels reported in literature.17a, 31 This could be explained by the measurement method: While the hydrodynamic diameter of NG is mostly determined by DLS in the free-floating state, the size measurements under the microscope are likely influenced by surface interactions between the glass slide and sample. The charged NG are pH- and ionic strength responsive, while neutral PNIPAAm NG only collapse at high ionic strengths (Figure S3, (A)). Given our aim of designing a gel system capable of orthogonal drug delivery, the NG must remain immobilized in the MG network under varying environmental conditions. To investigate pH- and ionic strength responsive behavior of the polyampholyte NiM−C, the samples were dried on an object slide and then rehydrated in a variety of buffers and salt solutions. The CLSM images show that the coacervates remain phase-separated at all measured pH values, 2.5 to 11.0, and at all salt concentrations, 1 mM to 1000 mM (Figure S13). As nNG are deswollen at pH values below pKA(MAAc)≈5.0 and pNG shrink at pH values above pKA(APMH)≈10.0 (Figure S3, S4), we verified that, even in the deswollen state, NG are immobilized in the MG network. Since PNIPAAm itself is ionic strength responsive, the entire NiM−C shrinks at 1000 mM due to the competition between solute ions and polymer chains for solvation. As electrolyte-water interactions substitute polymer-water interactions, the hydrophobic effect dominates and the MG networks collapse.14b Overall, the macro-responsivity of the polyampholyte NiM−C is similar to the responsivity of pure PNIPAAm MG (Figure 3, Figure S12–S14). Since swelling and deswelling of the NG does not affect the entire NiM−C, the neutral MG network appears to act like a sponge, compensating changes in NG size. The NiM−C morphology is stable at all measured pH values and at ionic strengths <1000 mM. Considering the ionic strength present in the human body (100–200 mM), the NiM−C system proves advantageous for biomedicine compared to free polyampholyte NG.40 To study the coacervation mechanism, microfluidic experiments were performed, in which water-in-oil droplets only contained certain reactants. CLSM images were taken of each sample before and after UV irradiation (Figure 4). With the identical droplet-based microfluidic setup as before, we first fabricated aqueous droplets containing both types of NG. In accordance with previous observations (Figure S10), they did not coacervate, neither before nor after irradiation (Figure 4 (A), (F)). The same experiment was repeated once with the addition of only LAP and once with the addition of only NIPAAm (Figure 4 (B), (C)). In a fourth experiment, both NIPAAm and LAP were added to the NG dispersion and lastly, all reactants used for the synthesis of polyampholyte NiM−C were employed (Figure 4 (D), (E)). All CLSM images before UV irradiation show a uniform distribution of NG inside the aqueous droplets, i.e. no coacervation occurred. The samples containing both NG species and either monomer or initiator did not exhibit coacervation even after UV irradiation (Figure 4 (G), (H)). Phase-separation was only observed for the sample that contains both types of NG with monomer as well as initiator, and the sample that additionally contains the crosslinker, both after UV irradiation and thus, radical decomposition of LAP (Figure 4 (I), (J)). The presence of both monomer and initiator in the droplets allows for polymerization. Hence, coacervation only occurs when (1) both positively and negatively charged NG species are present (Figure 2) and (2) linear or crosslinked polymers are formed. We conclude that—in addition to electrostatic interaction—depletion flocculation is an important driving factor for the assembly of oppositely charged polyelectrolyte NG. In short, a depletion zone exists around the NG that cannot be penetrated by the generated polymer chains. When the NG phase-separate, their respective depletion zones overlap, so that the free volume of the polymers is maximized.41 If only NG of the same charge are present during polymerization, this depletion force competes with the electrostatic repulsion between the NG,42 so the NG are statistically distributed throughout the MG network (Figure 2). However, with both NG species present, depletion force and electrostatic attraction prompt a cooperative-synergistic effect that triggers coacervation (Figure 4 (I), (J)). Hence, the introduction of charges is essential for the formation of compartments within NiM−C.
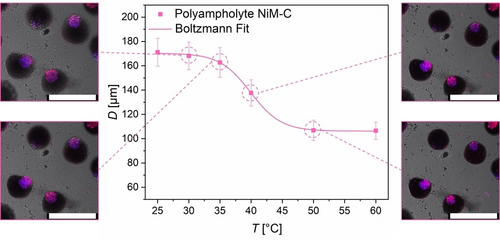
Influence of temperature on the hydrodynamic diameter of polyampholyte NiM−C in HPLC-grade water (pH ≈6.5) by optical microscopy. A sigmoidal Boltzmann fit gives VPTT≈40 °C. CLSM overlay images display polyampholyte NiM−C and their coacervates at 30, 35, 40 and 50 °C. Scale bars represent 300 μm.
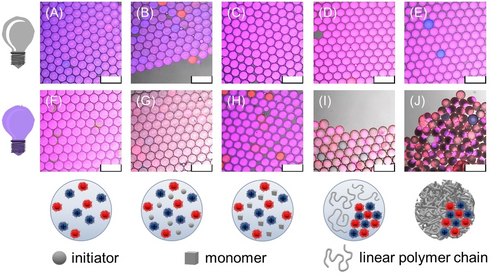
CLSM overlay images of water-in-oil droplets containing NG and different reactants before ((A)–(E)) and after UV irradiation ((F)–(J)). Below, schemes show the compounds within the aqueous droplets after UV irradiation. Coacervation only occurs after the generation of (mostly) linear (I) or crosslinked polymer chains (J). Scale bars represent 300 μm.
Based on this, we assume that the interaction dynamics would be significantly influenced by the synthesis parameters NG concentration, pH value and ionic strength. Thus, varying these parameters allows us to control internal morphology and NG arrangement within the NiM−C. First, the NG dispersion concentration used in the droplet-based microfluidic synthesis was varied between 5 and 30 mg/mL (Figure 5 (A)). The sample containing 10 mg/mL of each NG, which corresponds to the concentration used previously, showed the most pronounced phase-separation. In experiments in which the concentration was decreased to 5 mg/mL per NG dispersion, smaller coacervates formed. The smaller number of NG present in the droplets leads to a higher spatial separation within the MG network and thus, prevents the formation of one continuous coacervate. Upon increasing NG concentration, the size of the coacervate grows until it takes up most of the MG network. Surprisingly, at 30 mg/mL, the entire MG network is filled with NG exhibiting a core–shell like sub-structure, in which pNG are located in the interior and nNG are located at the periphery. For charge-stoichiometric solutions of oppositely charged polymers, it has already been demonstrated that above a certain concentration of polyelectrolytes, coacervation is suppressed and a single phase is formed.43 Based on our observation, we assume that, in this regard, the NG exhibit similar behavior to the linear polyelectrolyte chains. Additionally, recent studies suggest that the choice of co-monomer in PNIPAAm-based MG changes their interfacial activity.44 Based on the chemical structures of the MAAc and APMH moieties (Figure S7, S8), we assume that nNG are slightly more polar. Thus, we reason that the more hydrophilic nNG species is favored to align toward the hydrophilic surfactant head, while the slightly more hydrophobic pNG are more likely to be localized in the interior of the NiM−C. All three NG arrangements were examined by z-stacking CLSM images to verify their three-dimensional appearance (Figure S15).
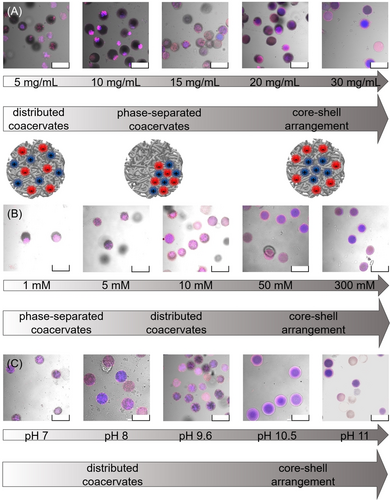
CLSM overlay images of polyampholyte NiM-C fabricated at different NG concentrations (A), ionic strengths (B) and pH values (C) during synthesis with a 1 : 1 NG ratio. NG concentrations of 10 mg/mL were used for the variation of ionic strength and pH value. Adjusting these parameters allows for the fabrication of NiM-C with phase-separated coacervates, statistically distributed coacervates and core-shell like arrangements of the NG. Scale bars represent 300 μm.
For the ionic strength variation, the NG (10 mg/mL) were redispersed in sodium chloride solutions with ionic strengths between 1 and 300 mM (Figure 5 (B)). As expected, phase-separation and coacervation were most pronounced in NiM−C obtained with a salt concentration of 1 mM. An increase in ionic strength resulted in less coacervation and a more homogeneous distribution of both NG species within the MG network. The higher the salt concentration is, the more the charges of the NG are screened. This in turn diminishes electrostatic attraction between the NG, which was hypothesized to be one of the driving forces of coacervation. The CLSM images in Figure 5 (B) verify this assumption. At salt concentrations of ≥50 mM, the charges are mostly screened and the polyelectrolyte NG deswell (Figure S3 (A)). Again, core–shell like arrangements of NG form inside the NiM−C. Following our hypothesis, we argue that the location of the NG at high salt concentrations is solely influenced by their interfacial activity and hydrophobicity, since electrostatic attraction is eliminated by charge screening.
To prove the effect of pH value on the internal structure of the NiM−C, the ionic strength was kept constant by employing buffers with ionic strengths of 10 mM.45 Although the buffers were specifically prepared with these low ionic strengths, the amount of free ions is sufficient to cause screening of some charges within the NG in the NiM−C. Of course, the screening is only effective proportionally to the ionic strength, but even so, it is enough to cause the coacervates to dissipate in the neutral pH range. Thus, the findings have to be compared rather to the experiments performed at an ionic strength of 10 mM than to those performed in HPLC-grade water (Figure 5). As the pKA of MAAc and APMH suggest and DLS and ELS substantiated (Figure S3, S4), the nNG are uncharged at pH <4.0 whereas the pNG are uncharged at pH >10.0. Due to the precipitation of the nNG in acidic buffers upon addition of NIPAAm and BIS, pH values <5.0 could not be tested. Possibly, the nNG are already largely protonated at pH 5.0 and thus, precipitate due to hydrophobic effects that occur in the presence of NIPAAm and BIS. Figure 5 (C) shows that at pH 7.0–9.6, smaller coacervates are spatially distributed throughout the NiM−C. The partial charge screening (I=10 mM) and increasing protonation of the APMH moieties result in a less pronounced phase-separation. At pH values 10.5 and 11.0, only nNG are charged. As only the electrostatic repulsion between negative charges prevails whereas attractive forces are diminished, core–shell like arrangements form.
Since Weitz et al. showed that thermoresponsive NIPAAm-based MG precipitate in aqueous droplets when heated above VPTT, temperature—in addition to electrostatic interactions and depletion flocculation—plays a role in the coacervation of MG/NG.28 Further experiments should be directed towards understanding the influence of the reaction temperature on NG coacervation. Additionally, NiM−C with core–shell like arrangements should be investigated to verify that hydrophobicity and interfacial activity of the NG are crucial. In relation to our aim of designing a drug delivery system with oppositely charged compartments, a deeper understanding of the decisive interactions provides control over NiM−C architecture and its mode of action. Preliminary experiments validate uptake and triggered release of a charged model drug46, 47 from NiM−C (Figure S16, S17). However, further optimization and analysis are needed to enhance the efficiency of absorption and expulsion of the cargo molecule and verify applicability for multiple drugs. Since the NiM−C are fabricated by a two-step approach, the size of the NiM−C can, in principle, be adjusted by changing the channel diameter of the microfluidic chip. It is already established that with droplet-based microfluidics, microgels with diameters between 10 μm and 1000 μm can be fabricated.24 Depending on the desired size of NiM−C, the diameter of the NG has to be reduced as well by tuning reaction conditions in precipitation polymerization. Generally, for the selection of an appropriate size, the planned pharmaceutical formulation is decisive: While an ideal size of 100–500 nm has been specified for drug delivery systems that are injected intravenously into the bloodstream,48 inhalable drug carriers, for example, should be between 0.5–5 μm in size.49 As the presented NiM−C exhibit unique pH-responsivity, administration by oral ingestion for targeted drug release in the gastrointestinal tract is conceivable. However, other therapeutic dosage forms such as transdermal intake could also be tested.50 In any case, size, surface functionalization and degradability of the NiM−C need to be optimized to improve their properties in vivo.48
Conclusion
In this work, we established a facile step-by-step synthesis route for the fabrication of polyelectrolyte and polyampholyte nanogel-in-microgel colloids (NiM−C). Confocal laser scanning microscopy was employed to visualize localization and arrangement of the NG within the NiM−C. Variation of pH value and ionic strength after the formation of the MG network proved that the morphology of the NiM−C remains intact under diverse environmental conditions, and that the NG are firmly immobilized in the MG network. We made plausible that the combination of electrostatic attraction between differently charged NG and depletion forces caused by the generation of polymer chains are the major driving forces for coacervation and phase-separation of the NG. Accordingly, careful selection of the synthesis parameters NG dispersion concentration, pH value and ionic strength allows for control over the internal morphology of the NiM−C to yield phase-separated or statistically distributed NG coacervates as well as core–shell like arrangements of the NG. Phase-separated coacervates were only obtained in HPLC-grade water (pH≈6.5) at low ionic strengths (I <5 mM) and a NG concentration of 10 mg/mL.
Acknowledgments
The authors acknowledge funding from the Deutsche Forschungsgemeinschaft (DFG) within Collaborative Research Center CRC 985 “Functional Microgels and Microgel Systems” (project A3), and thank the organization for the financial support. The authors gratefully acknowledge the support of Stefan Hauk in recording SEM images. Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.