Unlocking Migratory Insertion in Gold Redox Catalysis
Wenliang Wang
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorMeiling Ding
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorDr. Chuan-Gang Zhao
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorShuai Chen
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorProf. Dr. Chengjian Zhu
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
Green Catalysis Center, and College of Chemistry, Zhengzhou University, Zhengzhou, Henan, 450001 China
Search for more papers by this authorDr. Jie Han
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorDr. Weipeng Li
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Jin Xie
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
State Key Laboratory of Chemistry and Utilization of Carbon Based Energy Resources, College of Chemistry, Xinjiang University, Urumqi, 830017 China
Search for more papers by this authorWenliang Wang
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorMeiling Ding
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorDr. Chuan-Gang Zhao
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorShuai Chen
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorProf. Dr. Chengjian Zhu
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
Green Catalysis Center, and College of Chemistry, Zhengzhou University, Zhengzhou, Henan, 450001 China
Search for more papers by this authorDr. Jie Han
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorDr. Weipeng Li
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Jin Xie
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
State Key Laboratory of Chemistry and Utilization of Carbon Based Energy Resources, College of Chemistry, Xinjiang University, Urumqi, 830017 China
Search for more papers by this authorGraphical Abstract
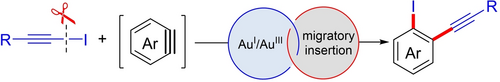
A gold(I)-catalyzed iodo-alkynylation of benzyne involving the merging of challenging migratory insertion and an oxidative addition process in the AuI/AuIII catalytic cycle has been reported. It represents important progress within the field of gold redox couple chemistry and in particular in the migratory insertion topic.
Abstract
Exploration of elementary reactions in organometallic catalysis is an important method with which to discover new reactions. In this article, we report a gold(I)-catalyzed iodo-alkynylation of benzyne involving the merging of challenging migratory insertion and an oxidative addition process in gold catalytic cycle. A wide range of structurally diverse alkynyl iodides are good coupling partners in this iodo-alkynylation transformation. Both aliphatic and aromatic alkynyl iodides can react with benzynes smoothly to afford highly functionalized 1,2-disubstituted aromatics in moderate to good yields. Its good functional group compatibility and late-stage application of complex molecules demonstrate its synthetic robustness. Studies of the mechanism reveals the feasibility of oxidative addition and the DFT calculations demonstrate the possible migratory insertion of benzyne into AuIII-carbon bonds in the AuI/AuIII redox catalytic cycle, representing an important step towards an elementary reaction in gold chemistry research.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202304019-sup-0001-misc_information.pdf10.3 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aR. H. Crabtree, The Organometallic Chemistry of the Transition Metals, 4th, Wiley, Hoboken, 2005;
- 1bJ. F. Hartwig, Organotransition Metal Chemistry From Bonding to Catalysis, University Science Books, Sausalito, 2010.
- 2M. Joost, A. Amgoune, D. Bourissou, Angew. Chem. Int. Ed. 2015, 54, 15022–15045.
- 3
- 3aA. S. K. Hashmi, G. J. Hutchings, Angew. Chem. Int. Ed. 2006, 45, 7896–7936;
- 3bC. M. Hendrich, K. Sekine, T. Koshikawa, K. Tanaka, A. S. K. Hashmi, Chem. Rev. 2021, 121, 9113–9163;
- 3cV. W. Bhoyare, A. G. Tathe, A. Das, C. C. Chintawar, N. T. Patil, Chem. Soc. Rev. 2021, 50, 10422–10450.
- 4D. Campeau, D. F. Leon Rayo, A. Mansour, K. Muratov, F. Gagosz, Chem. Rev. 2021, 121, 8756–8867.
- 5For selected examples:
- 5aT. Yuan, Q. Tang, C. Shan, X. Ye, J. Wang, P. Zhao, L. Wojtas, N. Hadler, H. Chen, X. Shi, J. Am. Chem. Soc. 2021, 143, 4074–4082;
- 5bX. Ye, H. Peng, C. Wei, T. Yuan, L. Wojtas, X. Shi, Chem 2018, 4, 1983–1993;
- 5cY. Yang, P. Antoni, M. Zimmer, K. Sekine, F. F. Mulks, L. Hu, L. Zhang, M. Rudolph, F. Rominger, A. S. K. Hashmi, Angew. Chem. Int. Ed. 2019, 58, 5129–5133;
- 5dX. Li, X. Xie, N. Sun, Y. Liu, Angew. Chem. Int. Ed. 2017, 56, 6994–6998.
- 6
- 6aD. A. Roşca, D. A. Smith, D. L. Hughes, M. Bochmann, Angew. Chem. Int. Ed. 2012, 51, 10643–10646;
- 6bM. Joost, P. Gualco, S. Mallet-Ladeira, A. Amgoune, D. Bourissou, Angew. Chem. Int. Ed. 2013, 52, 7160–7163;
- 6cM. W. Johnson, S. W. Bagley, N. P. Mankad, R. G. Bergman, V. Mascitti, F. D. Toste, Angew. Chem. Int. Ed. 2014, 53, 4404–4407;
- 6dM. Joost, L. Estevez, S. Mallet-Ladeira, K. Miqueu, A. Amgoune, D. Bourissou, J. Am. Chem. Soc. 2014, 136, 10373–10382;
- 6eF. Rekhroukh, R. Brousses, A. Amgoune, D. Bourissou, Angew. Chem. Int. Ed. 2015, 54, 1266–1269;
- 6fD. A. Rosca, J. Fernandez-Cestau, J. Morris, J. A. Wright, M. Bochmann, Sci. Adv. 2015, 1, e1500761;
- 6gA. Pintus, L. Rocchigiani, J. Fernandez-Cestau, P. H. Budzelaar, M. Bochmann, Angew. Chem. Int. Ed. 2016, 55, 12321–12324;
- 6hF. Rekhroukh, C. Blons, L. Estevez, S. Mallet-Ladeira, K. Miqueu, A. Amgoune, D. Bourissou, Chem. Sci. 2017, 8, 4539–4545;
- 6iJ. Fernandez-Cestau, L. Rocchigiani, A. Pintus, R. J. Rama, P. H. M. Budzelaar, M. Bochmann, Chem. Commun. 2018, 54, 11447–11450;
- 6jL. Rocchigiani, J. Fernandez-Cestau, I. Chambrier, P. Hrobarik, M. Bochmann, J. Am. Chem. Soc. 2018, 140, 8287–8302;
- 6kJ. Serra, P. Font, E. D. Sosa Carrizo, S. Mallet-Ladeira, S. Massou, T. Parella, K. Miqueu, A. Amgoune, X. Ribas, D. Bourissou, Chem. Sci. 2018, 9, 3932–3940;
- 6lA. V. Zhukhovitskiy, I. J. Kobylianskii, C. Y. Wu, F. D. Toste, J. Am. Chem. Soc. 2018, 140, 466–474;
- 6mJ. A. Cadge, P. J. Gates, J. F. Bower, C. A. Russell, J. Am. Chem. Soc. 2022, 144, 19719–19725.
- 7
- 7aE. Langseth, A. Nova, E. A. Traseth, F. Rise, S. Oien, R. H. Heyn, M. Tilset, J. Am. Chem. Soc. 2014, 136, 10104–10115;
- 7bM. S. M. Holmsen, C. Blons, A. Amgoune, M. Regnacq, D. Lesage, E. D. Sosa Carrizo, P. Lavedan, Y. Gimbert, K. Miqueu, D. Bourissou, J. Am. Chem. Soc. 2022, 144, 22722–22733.
- 8
- 8aW. Li, D. Yuan, G. Wang, Y. Zhao, J. Xie, S. Li, C. Zhu, J. Am. Chem. Soc. 2019, 141, 3187–3197;
- 8bK. Liu, N. Li, Y. Ning, C. Zhu, J. Xie, Chem 2019, 5, 2718–2730;
- 8cK. Liu, T. Li, D.-Y. Liu, W. Li, J. Han, C. Zhu, J. Xie, Sci. China Chem. 2021, 64, 1958–1963;
- 8dW. Wang, C.-L. Ji, K. Liu, C.-G. Zhao, W. Li, J. Xie, Chem. Soc. Rev. 2021, 50, 1874–1912;
- 8eS. Witzel, A. S. K. Hashmi, J. Xie, Chem. Rev. 2021, 121, 8868–8925;
- 8fC.-L. Ji, J. Han, T. Li, C.-G. Zhao, C. Zhu, J. Xie, Nat. Catal. 2022, 5, 1098–1109;
- 8gX. Xia, J. Xie, Gold Bull. 2022, 55, 123–127;
- 8hH. Liang, Y. Julaiti, C.-G. Zhao, J. Xie, Nat. Synth. 2023, 2, 338–347.
- 9
- 9aZ. Xia, V. Corce, F. Zhao, C. Przybylski, A. Espagne, L. Jullien, T. Le Saux, Y. Gimbert, H. Dossmann, V. Mouries-Mansuy, C. Ollivier, L. Fensterbank, Nat. Chem. 2019, 11, 797–805;
- 9bF. Zhao, M. Abdellaoui, W. Hagui, M. Ballarin-Marion, J. Berthet, V. Corce, S. Delbaere, H. Dossmann, A. Espagne, J. Forte, L. Jullien, T. Le Saux, V. Mouries-Mansuy, C. Ollivier, L. Fensterbank, Nat. Commun. 2022, 13, 2295.
- 10For earlier works related to migratory insertion to arynes in gold catalysis, see the following selection:
- 10aC. Xie, Y. Zhang, Y. Yang, Chem. Commun. 2008, 4810–4812;
- 10bK. Sato Menggenbateer, T. Kubota, N. Asao, Tetrahedron 2008, 64, 787–796; For aryne-like intermediates being involved in gold catalysis, see for instance:
- 10cM. M. Hansmann, S. Tsupova, M. Rudolph, F. Rominger, A. S. K. Hashmi, Chem. Eur. J. 2014, 20, 2215–2223;
- 10d“Arylation Reactions involving the formation of arynes”: Y. Chen, R. C. Larock in Modern Arylation Methods, Wiley-VCH, Weinheim, 2009, pp. 401–473;
10.1002/9783527627325.ch12 Google Scholar
- 10eJ. Shi, L. Li, Y. Li, Chem. Rev. 2021, 121, 3892–4044.
- 11Y. Himeshima, T. Sonoda, H. Kobayashi, Chem. Lett. 1983, 12, 1211–1214.
- 12
- 12aL. Zhang, C. Wei, J. Wu, D. Liu, Y. Yao, Z. Chen, J. Liu, C. J. Yao, D. Li, R. Yang, Z. Xia, Chem. Sci. 2022, 13, 7475–7481;
- 12bJ. Wu, C. Wei, F. Zhao, W. Du, Z. Geng, Z. Xia, J. Org. Chem. 2022, 87, 14374–14383;
- 12cJ. Xie, S. Shi, T. Zhang, N. Mehrkens, M. Rudolph, A. S. K. Hashmi, Angew. Chem. Int. Ed. 2015, 54, 6046–6050;
- 12dW. Cao, S.-L. Niu, L. Shuai, Q. Xiao, Chem. Commun. 2020, 56, 972-975.
- 13
- 13aA. Zeineddine, L. Estevez, S. Mallet-Ladeira, K. Miqueu, A. Amgoune, D. Bourissou, Nat. Commun. 2017, 8, 565;
- 13bM. Rigoulet, O. Thillaye du Boullay, A. Amgoune, D. Bourissou, Angew. Chem. Int. Ed. 2020, 59, 16625–16630;
- 13cC. C. Chintawar, A. K. Yadav, N. T. Patil, Angew. Chem. Int. Ed. 2020, 59, 11808–11813;
- 13dS. Zhang, C. Wang, X. Ye, X. Shi, Angew. Chem. Int. Ed. 2020, 59, 20470–20474.
- 14For gold catalyst L6AuCl: palladium, copper and nickel contaminant cannot be detected by ICP-OES analysis. We would like to thank the referees very much for having suggested this check.
- 15Deposition Number 2225167 (for 12) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 16
- 16aA. S. K. Hashmi, Angew. Chem. Int. Ed. 2010, 49, 5232–5241;
- 16bT. Lauterbach, A. M. Asiri, A. S. K. Hashmi, Adv. Organomet. Chem. 2014, 62, 261–297.
- 17Hashmi and co-workers reported a gold-catalyzed coupling through oxidative addition of bromoalkynes in 2019:
- 17aY. Yang, J. Schiessl, S. Zallouz, V. Goker, J. Gross, M. Rudolph, F. Rominger, A. S. K. Hashmi, Chem. Eur. J. 2019, 25, 9624–9628;
- 17bJ. A. Cadge, H. A. Sparkes, J. F. Bower, C. A. Russell, Angew. Chem. Int. Ed. 2020, 59, 6617–6621;
- 17cJ. Rodriguez, A. Tabey, S. Mallet-Ladeira, D. Bourissou, Chem. Sci. 2021, 12, 7706–7712.
- 18C. X. Liu, P. P. Xie, F. Zhao, Q. Wang, Z. Feng, H. Wang, C. Zheng, S. L. You, J. Am. Chem. Soc. 2023, 145, 4765–4773.
- 19
- 19aY. Li, D. Qiu, R. Gu, J. Wang, J. Shi, Y. Li, J. Am. Chem. Soc. 2016, 138, 10814–10817;
- 19bX. Xiao, T. Wang, F. Xu, T. R. Hoye, Angew. Chem. Int. Ed. 2018, 57, 16564–16568.
- 20
- 20aK. P. Kepp, J. Phys. Chem. A 2017, 121, 2022–2034;
- 20bM. H. Larsen, K. N. Houk, A. S. K. Hashmi, J. Am. Chem. Soc. 2015, 137, 10668–10676;
- 20cK. Wang, X. Bao, J. Org. Chem. 2023, 88, 1107–1112.