Modern Strategies for Carbon Isotope Exchange
Graphical Abstract
The field of carbon isotope labeling has recently explored new alternative avenues for better dealing with the hurdles of radiochemistry. This review focuses on the emerging opportunities provided by carbon isotope exchange (CIE) technologies. By dramatically reducing the number of steps and radioactive waste generated in preparation of tracers, CIE has great potential for accelerating clinical development of pharmaceuticals.
Abstract
In contrast to stable and natural abundant carbon-12, the synthesis of organic molecules with carbon (radio)isotopes must be conceived and optimized in order to navigate through the hurdles of radiochemical requirements, such as high costs of the starting materials, harsh conditions and radioactive waste generation. In addition, it must initiate from the small cohort of available C-labeled building blocks. For long time, multi-step approaches have represented the sole available patterns. On the other side, the development of chemical reactions based on the reversible cleavage of C−C bonds might offer new opportunities and reshape retrosynthetic analysis in radiosynthesis. This review aims to provide a short survey on the recently emerged carbon isotope exchange technologies that provide effective opportunity for late-stage labeling. At present, such strategies have relied on the use of primary and easily accessible radiolabeled C1-building blocks, such as carbon dioxide, carbon monoxide and cyanides, while the activation principles have been based on thermal, photocatalytic, metal-catalyzed and biocatalytic processes.
1 Introduction
The insertion of isotopic labels onto organic molecules is a well-recognized tool to track the faith of synthetic organic compounds and unveil their behavior both in the environment and in vitro or in vivo.1 The choice of a specific isotope is dictated by a multiplicity of factors, such as the energy of particles emitted over its radioactive decay, its half-life, the type of application and, in case where multiple options are available, the ease of synthetic access to the desired radiotracer. As carbon is omnipresent and at the foundation of life on earth, the insertion of carbon isotope label(s) into organic compounds has received extensive attention over the past century, but has heavily relied on lengthy multi-step processes.2 In contrast to deuterium and tritium labeling, revolutionized by extensive efforts on C−H bond activation and Hydrogen Isotope Exchange (HIE) technologies,3 the direct replacement of carbon isotopes into organic molecules has long remained elusive. The advent of Carbon Isotope Exchange (CIE) has only recently emerged as a concrete alternative for carbon labeling, and stepped its isotopes into a new age of opportunities (Scheme 1). Since 2018, modern examples of CIE have appeared and concrete proof-of-concepts were obtained with carbon-14 (14C, β− emitter, half-life 5730 years), as well as carbon-11 (11C, β+ emitter, half-life 20.4 minutes).
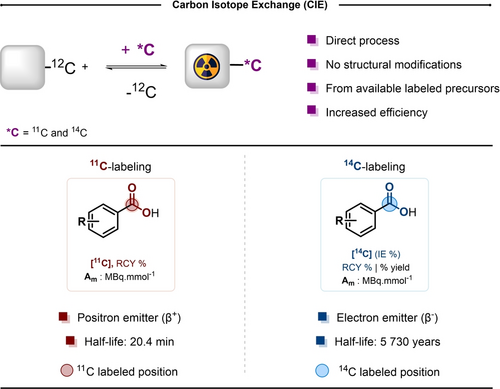
Concept of carbon isotope exchange (CIE). RCY: Radiochemical yield. Am: molar activity. IE : Isotope Enrichment.
This review will briefly highlight the progress in this vibrant state of the art, with a special focus on the methods that allow for carbon isotope replacement in one single step, under dynamic conditions. In addition, some representative examples of isotope replacement, by means of a deconstruction/reconstruction strategies applied to radiocarbon, will be highlighted, as well.
2 Carbon Isotope Exchange on Carboxylic Acids
Nowadays, 14C is artificially generated in a nuclear reaction as Ba[14C]CO3, the unique precursor from which all 14C-labeled molecules originate. Consequently, efforts towards late-stage labeling and CIE have thus far largely focused on the use of [14C]CO2, as a primary isotope source, and targeted carboxylic acids as functional groups.
2.1 Metal Catalyzed Exchange
Copper (I) is known to be an effective catalyst for decarboxylative cross-coupling reactions4 and it has been also well-described for its propensity to promote CO2 fixation reactions from boronic acids.5 Based on these precedents, in 2019, Cantat and Audisio reported a CIE technology using copper(I) bromide with bisoxazoline (BOX) ligands in dimethyl sulfoxide (DMSO) at 150 °C, to label (hetero)aromatic carboxylic acids in presence of 3 equivalents of isotopically labeled CO2 (Scheme 2).6 It is worth mentioning that since one equivalent of [12C]CO2 was generated during the decarboxylation step, the theoretical 12C/13,14C ratio in the reaction mixture cannot exceed 25/75. Under such conditions, the isotopic enrichment (IE) on the model substrate was found to be 75 %, which corresponds to the maximal theoretical value possible under this stoichiometry. In the reaction, the use of cesium salt of the corresponding acids was required, in order to limit the formation of the proto-decarboxylation byproducts. The procedure proved suitable for a variety of carboxylates, including heteroaryl derivatives, such as benzofurans, coumarins and thiophenes. On the other hand, it was unsuitable for substrates bearing electrodonating methoxy substituents or containing labile protons, which underwent proto-decarboxylation. The CIE was conveniently applied to both 13C and 14C labeling of pharmaceutically active compounds. Probenecid [14C]1, Flumequine [14C]2 and Febuxostat [14C]3 were radiolabeled in 40 % to 54 % IE, corresponding to molar activities (Am) between 923 and 1250 MBq mmol−1, fully in line with applications for ADME studies. Nonetheless, given the three fold excess of [14C]CO2, radiochemical yields (RCY, i.e. based on the radiolabeled reagent) are low.
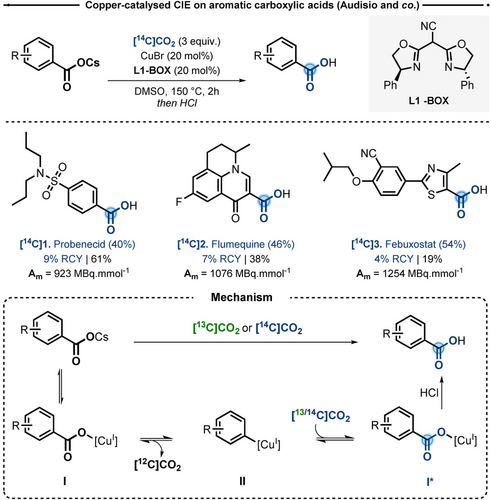
Top) Late-stage copper-catalyzed CIE of (hetero)aromatic carboxylic acids. Bottom) Proposed reaction mechanism. Box=Bisoxazoline
The proposed mechanism involved the formation of a copper carboxylate intermediate (I, Scheme 2), which underwent thermal decarboxylation at 150 °C to form a transient Cu-Csp2 species (II, Scheme 2). Subsequent insertion of the carbon-labeled CO2 would form the labeled intermediate (I*, Scheme 2). Finally, after protonation, the desired C-labeled carboxylic acid was isolated.
Carbon monoxide (CO) is a valuable C1-building block, which displays high versatility in a large array of chemical reactions, particularly in the area of transition-metal-catalyzed carbonylative cross-couplings.7 In contrast with CO2, which is thermodynamically more stable and a common building block in radiochemistry,8 [11C]CO and [14C]CO isotopologues are less trivial reagents. For [14C]CO, its radiolysis prevents long-term storage and, in addition, to authority control, waste and safety precautions renders its manipulation more complicated.9 On the other hand, the challenging synthetic access to [11C]CO, the need for automation and the short half-life limit its utilization to only selected research centers. In 2018, Gauthier et al. explored a palladium-catalyzed CO isotope exchange starting from activated acid chloride derivatives (Scheme 3).10
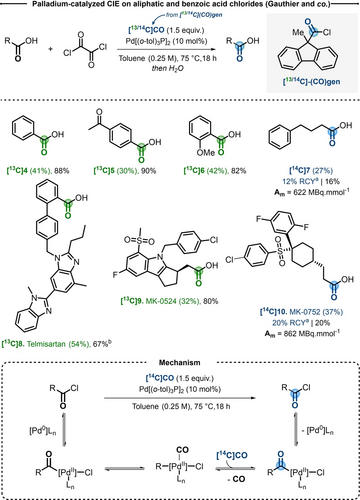
Top) Selected examples of the substrate scope for CIE using [13/14C](CO)gen. a) As reported by the authors in the original paper. b) N-methyl-2-pyrrolidone (NMP) instead of toluene. Bottom) Proposed reaction mechanism.
The optimization of the reaction was performed on model substrate dihydro-cinnamoyl chloride, under excess of [13C]CO (1.5 atm), highlighting Pd2dba3 (10 mol %) and tri(o-tolyl)phosphine (20 mol %) as optimal catalytic system to provide high 13C-incorporation, at the same time limiting the competitive β-hydride elimination side reaction. To apply this technology to 13C and 14C-labeling, the use of [13/14C]COgen was next investigated. COgen is a solid CO surrogate developed by the group of Skrydstrup, which allows to generate in situ precise amounts of CO in a two-chamber reaction glassware.11 With a stoichiometry of 1.5 equivalents with respect to the acid chloride, a series of aromatic and primary aliphatic derivatives were labeled in moderate to good yields and isotope incorporations. Several biologically active substrates, such as Telmisartan [13C]8 and MK-0524 [13C]9 were successfully labeled. As radioactive proof-of-concept, MK-0752 [14C]10 was radiolabeled, using [14C]COgen, in 37 % IE corresponding to 862 MBq mmol−1 (Am) (Scheme 3). Since the standard Pd/P(o-tol)3 conditions did not perform effectively in presence of secondary and tertiary aliphatic acids, further catalyst screening showcased that bulky and electron rich ligands such as tBu3P and JohnPhos could overcome such limitations. Remarkably, at room temperature, chiral (S)-ibuprofene chloride could be obtained in 32 % IE, without loss of stereoinformation or isomerization. While the scope of this reaction was remarkably broad, current limitations were the requirement to use sensitive acid chloride derivatives and the need for a sophisticated and more expensive isotopic source of [14C]CO.
In 2019, the groups of Baran and Martin independently reported a nickel-mediated CIE procedure to access to C-labeled aliphatic carboxylic acids, starting from the corresponding N-hydroxyphthalimide (NHPI) Redox-Active Esters (RAE).12, 13 Optimized conditions allowed to isolate labeled 5-phenylvaleric acid in 42 % yield and a moderate 19 % IE, in presence of a large excess of [13C]CO2 (3.4 bar), using stoichiometric amounts of NiBr2.glyme (1.0 equiv), neocuproine (2.2 equiv) and manganese (2.2 equiv) in N,N-dimethylformamide (DMF) at room temperature. With such conditions, ten RAE derivatives were labeled in 8 to 50 % IE with 13C, but in low to moderate yields (8 to 40 %). Unfortunately, tertiary carboxylic acids remained challenging. It is worth mentioning that the complexity of the substrate scope is large and functional groups tolerance including ketones, esters, amides, phenols and protected amines is of note (Scheme 4). In order to adapt this methodology to radioactive 14C, a slight modification was implemented. The reactions was cooled at −25 °C, to improve the solubility of [14C]CO2 in the solvent14 and a controlled excess of the gas (5.5 to 32.0 equiv) was utilized. The 14C-procedure was applied to five molecules, including [14C]Mycophenolic acid [14C]15 and [14C]Chlorambucil [14C]16 synthesized in 48 % and 38 % yield and 466 and 384 MBq mmol−1 Am, respectively. Though the isotope incorporation and RCY were moderate, this reversible carboxylation was significantly more effective compared to the previously reported multistep radiosynthesis.15, 16
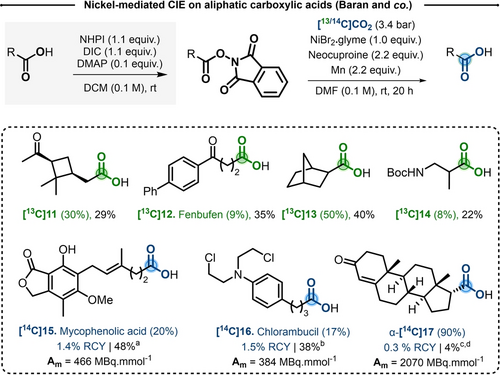
Selected examples for Ni-mediated CIE on aliphatic carboxylic acids. NHPI=N-hyroxyphthalimide. DIC=N,N′-diisopropylcarbodiimide. DMAP=4-dimethylaminopyridine. a) [14C]CO2: 8.6 equiv. b) [14C]CO2: 5 equiv. c) [14C]CO2: 19.2 equiv. d) Both diastereomers were formed during the reaction but the alpha was isolated with a higher Am.
The protocol developed by Martin and co-workers used catalytic amount of NiCl2.dme (10 mol %), with substituted bipyridine L2 (25 mol %) and Mn (2.0 equiv) in DMF:MeOH (3 : 1) under an atmospheric pressure of [13C]CO2 at 0 °C. Starting from the corresponding NHPI ester, octanoic acid [13C]18 was obtained in 55 % yield and 56 % IE. The substrate scope showed the compatibility with nitriles, alkenes, carbamates, or nitrogen containing heterocycles (Scheme 5). Biologically relevant molecules such as the Citronellic acid [13C]23 and Lithocholic acid [13C]24 were labeled in good yields and 48 % to 65 % IE.
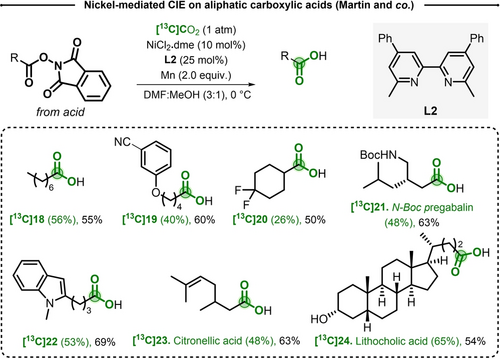
Selected examples for Ni-mediated CIE on NHP ester pre-functionalized aliphatic carboxylic acid. L2=6,6′-dimethyl-4,4′-diphenyl-2,2′-bipyridine.
2.2 Thermal Exchange
Though early examples of CIE on alkyl carboxylic acids date back to the 60s,17 the harsh thermal (T>300 °C) or pyrolytic conditions reported prevented concrete applications. On the other hand, in 1978, Parnes reported a methodology suitable for isotope exchange of carboxylic acid 25.18 Treatment of the acid with excess Lithium Diisopropylamide (LDA) in Hexamethylphosphoramide (HMPA) allowed to deprotonate the acid and generate an anion in alpha position to the carboxylate, which was further reacted with [14C]CO2 to form transient malonate intermediate (Int, Scheme 6). After decarboxylation, the labeled product [14C]25 was obtained in 48 % RCY and modest IE (14 %). Despite the modest isotope incorporation and the need of strong base and HMPA, this result should be considered in its relevance for further developments in the field.
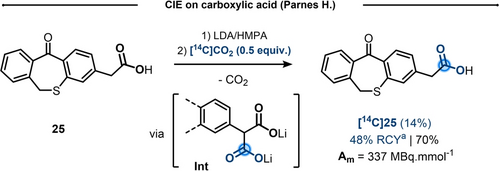
CIE on carboxylic acid using a malonate intermediate. a) As reported by the author in the original paper.
Among pharmaceutically active carboxylic acids, the Phenyl Acetic Acid (PAA) scaffold occupies a predominant place in the Non-Steroidal Anti-Inflammatory Drugs (NSAID). Among such medicines, widely used to relieve pain and inflammation, several notorious examples such as ibuprofen, naproxen, ketoprofen and diclofenac can be recalled. In this context, in 2020, the groups of Audisio and of Lundgren reported two concomitant strategies for carboxylate isotope replacement.
The group of Lundgren published a reversible decarboxylation process on PAA and malonates, without need for additional pre-functionalization.19 This procedure was implemented from potassium carboxylates in presence of excess of [13C]CO2 (1 atm) in DMF and it allowed to obtain the labeled acids in high isotopic incorporation, in a single step. Interestingly, the electronic nature of substituents on the aromatic ring was found to substantially affect the rate of the transformation. While (hetero)arylacetic acid salts with anion-stabilizing groups underwent exchange at moderate temperatures (PPA with 4-CN, and 2-NO2 substitutions were fully exchanged at room temperature), substrates bearing electron donating substituents required high temperatures (Scheme 7). As such, to label a series of NSAID, such as Naproxen [13C]30, Zomepirac [13C]31 and Ibuprofen [13C]32 (Scheme 7), a prolonged reaction time of 48 h at 160 °C in DMSO was required. While this technology had potential for implementation with radiocarbon, only stable [13C]CO2 was utilized in the work.
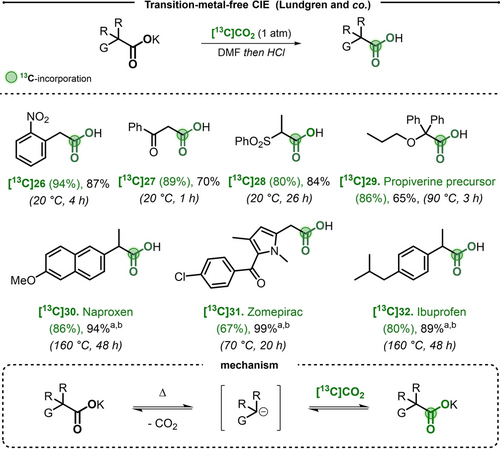
Top) Selected examples for CIE of arylacetates and  -substituted acetates.G=(hetero)aryl, ester, ketone, amide, sulfonyl, nitrile. R=aryle, alkyl, H. a) DMSO used instead of DMF. b) NMR yield. Bottom) Proposed reaction mechanism.
-substituted acetates.G=(hetero)aryl, ester, ketone, amide, sulfonyl, nitrile. R=aryle, alkyl, H. a) DMSO used instead of DMF. b) NMR yield. Bottom) Proposed reaction mechanism.
Independently, the same year Audisio and co-workers published a transition-metal-free CIE of PAA cesium carboxylates.20 In the experimental procedure, the exchange was performed in presence of a precise stoichiometry of labeled CO2 (3 equiv) in DMSO at 150 °C in two hours. The scope of the reaction was explored on a serie of 16 derivatives bearing commonly found electron-donating and electron-withdrawing groups. Importantly, a library of 13 pharmaceutically relevant PAA were labeled at temperatures between 150 °C and 190 °C for several examples. More interestingly, only 3.5 hours were required to label Ibuprofen [13C]35 in 33 % yield and 32 % IE. Simply by replacing [13C]CO2 by [14C]CO2, this method was implemented to the radiosynthesis of Felbinac [14C]36, Fenclofenac [14C]37 and Metiazinic acid [14C]38, providing average Am in the range of 1500 MBq mmol−1. In collaboration with the group of Schou at the Karolinska Institut, we described a first example of CIE on carbon-11 (11C). Using this short lived  emitter (T1/2=20.4 min), a proof-of-concept was obtained using [11C]CO2 during a short reaction time of 5 minutes. Indeed, 2-(4-nitrophenyl)acetic acid [11C]39 was successfully labeled and isolated in 11 % RCY providing a Am of 160 MBq
emitter (T1/2=20.4 min), a proof-of-concept was obtained using [11C]CO2 during a short reaction time of 5 minutes. Indeed, 2-(4-nitrophenyl)acetic acid [11C]39 was successfully labeled and isolated in 11 % RCY providing a Am of 160 MBq  mol−1 valuable for in vivo organ distribution studies (Scheme 8).
mol−1 valuable for in vivo organ distribution studies (Scheme 8).
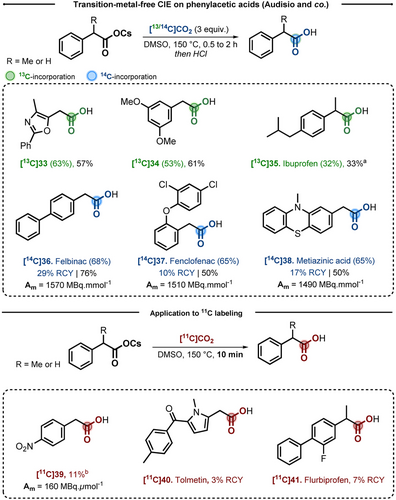
Transition-metal-free CIE on PAA pharmaceuticals and application for 11C, 13C and 14C. a) 30 min, 190 °C. b) 5 min, 80 °C.
Despite their importance, labeled amino acids remained essentially unexplored by modern CIE, until recently, when the groups of Rotstein and Lundgren published a novel, dynamic access to these compounds, based on a biomimetic approach involving the decarboxylation/carboxylation of imino acids.21 Phenylalanine could be enriched at 75 % and isolated in 84 % yield, in presence of 4-anisaldehyde (20 mol %), cesium carbonate (40 mol %) and [13C]CO2 (8 equiv). The investigation of the scope showcased a large function tolerance on proteinogenic and non-proteinogenic amino acids (bearing iodine, bromine, fluorine, azide, boronic acids …), with 13C-incorporation ranging from 19 to 84 %. On the other hand, substrates such as Boc-protected proline, histidine, threonine and cysteine provided 13C-incorporation inferior to 10 %. However, these limitations could be overcome by re-submitting the substrates to CIE. For instance, phenylalanine [13C]42 was enriched at 75 % after one cycle and 96 % after a second one. A trump card of this strategy was its adaptability to 11C-labeling under modified conditions. The imine carboxylate was pre-formed and reaction time reduced to 10 minutes. As such, phenylalanine [11C]49 was labeled with 24 % RCY and a Am of 8400 MBq mmol−1. This method draws inspiration from pyridoxal phosphate-catalyzed α-amino acid decarboxylation22 and would involve the following mechanistic steps: (a) condensation between the amino acid and the aldehyde led to the apparition of a Schiff base (I, Scheme 9); (b) deprotonation enables the formation of nucleophilic imine-enol intermediate (II, Scheme 9); (c) reversible carboxylation occurs conducting to an iminomalonate intermediate (III, Scheme 9). Inherent racemization of the amino acid was thus inevitable, but efforts have been made in order to obtain enantio-enriched products using known enzymatic or metal-catalyzed dynamic kinetic resolution approaches. In this manner L-[13C]phenylalanine, L-[13C]leucine and L-[13C]methionine have been obtained in high enantiomeric excess.

Aldehyde-catalyzed CIE on amino acids with labeled CO2. a) [14C]CO2 (2.6 equiv, 2310 MBq mmol−1), 17 h. b) catalyst (75 mol %), Cs2CO3 (1 equiv), 0.017 M. c) Isolated after Boc protection. d) Reactions were conducted using 7000–10 000 MBq [11C]CO2.
2.3 Photochemical Exchange
While thermal examples of CIE has opened new avenues for late-stage carbon labeling, conditions utilized to access to PAA still remained harsh (Schemes 7 and 8). In order to overcome such limitation, in 2021 the groups of Audisio and of Lundgren independently reported alternative photochemical strategies.
In the protocol reported by Audisio and co-workers, the exchange was performed from the corresponding carboxylic acid, without need for pre-functionalization, in presence of [13C]CO2, the organic photocatalyst 2,4,5,6-tetra(9H-carbazol-9-yl) isophthalonitrile (4CzIPN, 6 mol %), potassium phosphate (1 equiv) in DMF under LED blue-light irradiation during 6 hours (Scheme 10).23 The optimized conditions were accurately selected in order to apply the procedure to radiolabeling with 14C. As such, three equivalents of labeled CO2 were precisely added in the reactor and the inorganic phosphate base was preferred over carbonates, to avoid additional isotopic dilution.24 With more than 40 examples, the scope of the reaction showed generally moderate to good yields and IE in the range of 40 % to 70 %. Note that under such stoichiometry, the maximal IE was 75 %. Halogens, most common electron-donating and electro-withdrawing substituents on the aromatic ring were tolerated. In addition, PAA substituted in the alpha position to the carboxylic acid were compatible, including examples of N-protected non-natural aminoacids [13C]54. Importantly, this photocatalytic CIE was applied for the labeling of notorious pharmaceuticals, such as Naproxen [13C]56 and Flurbiprofen [13C]57, which previously required drastic thermal conditions (Scheme 7). The reaction was next accommodated for 14C-radiolabeling. In that respect, by delivering [14C]CO2 (via a RC-TRITEC® manifold), [14C]55 and Ibuprofen [14C]58 were radiolabeled in 47 and 29 % yield, with 62 and 53 % of IE leading to Am of 1434 and 1225 MBq mmol−1, respectively (Scheme 10).
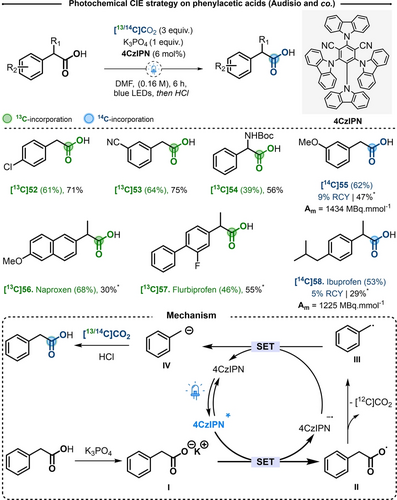
Top) Photochemical CIE strategy on phenylacetic acids. * 3 h. Bottom) Proposed reaction mechanism.
Based on a series of mechanistic experiments and on previous literature reports, a proposed reaction mechanism was suggested (Scheme 10).25 After irradiation of the photocatalyst 4CzIPN, oxidation of the in situ formed carboxylate takes place, leading to the formation of a benzylic radical (III) and the liberation of [12C]CO2. After a subsequent single electron transfer, anion IV was formed and may further trap labeled-carbon dioxide. Experiments in presence of stoichiometric amounts of [13C]CO2, have shown that with less than two equivalents of labeled electrophile proto-decarboxylation was the major product of the reaction. These experiments suggest that the reaction time and the amount of gas must be carefully taken into consideration.
The same year, Lundgren and co-workers reported a similar procedure to label primary and secondary PAA and α-substituted acetic acids. The authors used 5 mol % of 4CzIPN, cesium carbonate as base with one atmosphere of [13C]CO2 in DMSO under visible blue LED irradiation.26 Following this conditions, 26 arylacetic acids substituted by a wide array of electron-donating and electron-withdrawing groups and 7 examples of α-substitued acetic acids, including malonate derivatives, were successfully obtained in medium to excellent IE and yields. The authors showed that replacing 4CzIPN with (2,3,4,6)-3-benzyl-2,4,5,6-tetra(9H-carbazol-9-yl)benzonitrile (4CzBnBN, 5 mol %), an in situ formed photocatalyst previously reported by the group of König,27 was key to label tertiary carboxylic acids. Interestingly, the use of 4CzBnBN was effective on substrates where 4CzIPN failed and allowed to shorten the reaction time to only 10 minutes. Taking advantage of this opportunity the authors explored the CIE on short lived 11C. As such, Caprofen [11C]67, Felbinac [11C]68 and Fenoprofen [11C]69 were successfully radiolabeled in 18 to 37 % RCY using [11C]CO2 (Scheme 11).
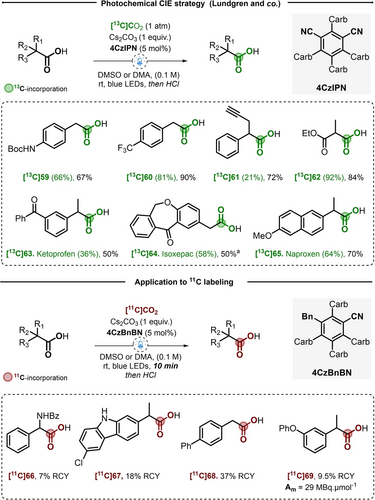
Photochemical CIE strategy on carboxylic acids. Carb=Carbazole. Bn=Benzyl. a) Percent 13C-incorporation and yield determined after conversion to the benzyl ester.
2.4 Enzyme Promoted CIE
Decarboxylation is one of the most common reactions in nature and represents a keystone of many biochemical processes.22, 28 As carboxylic acids are usually stable, their decarboxylation in biological environment requires the intervention of specific enzymes, decarboxylases, which promote the decarboxylation by stabilizing the transition states and, specifically, the residual carbanion by interactions with the enzyme and/or cofactors.29 On the other hand, carboxylations also occurs in nature and are responsible for the fixation of inorganic carbon (i.e. Calvin-Benson-Bassham cycle).30 Unfortunately, enzymes promoting CO2 fixation are usually different from those that contribute to the reverse reaction, the decarboxylation. Nevertheless, some exceptions are known. For example, the enzyme Aspergillus niger is implicated in non-oxidative reversible decarboxylation of aromatic substrates.31
In 1964, Benziman and co-workers reported that Acetobacter xylinum cells perform a [14C]CO2 exchange into Oxaloacetate (OAA).32, 33 OAA decarboxylase catalyzed the OAA decarboxylation into pyruvate, but also the OAA-[14C]CO2 exchange. Interestingly, it was highlighted that the exchange was occurring exclusively on the OAA β-carboxyl moiety. The presence of manganese or magnesium divalent cations appeared essential for the exchange. It was possible to determinate that, on a five minutes scale, the exchange activity was around 36 nmol min−1 mg−1 of protein (Scheme 12). Similarly, some OAA-[14C]CO2 exchange was highlighted to occur in extracts of Micrococcus lysodeikticus.34
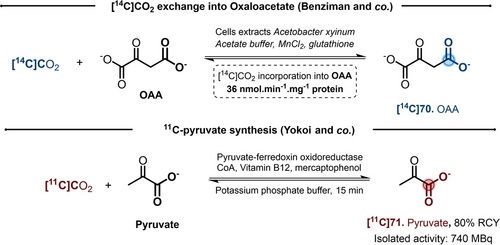
Pioneering examples of enzymatic catalyzed CIE.
Likewise, CO2 exchange on pyruvate is known in many cell-free extracts, and particularly in Clostridium butyricum and Clostridium cylindrosporum.35 In 1971, Raeburn and Rabinowitz pointed out the key role of Pyruvate-Ferredoxin Oxidoreductase (PFOR) in the process.36 In 1985, Yokoi and co-workers developed an enzyme catalyzed methodology for the synthesis of [11C]pyruvate.37 Despite the challenges related to this short-lived isotope,38 the authors were able to trap [11C]CO2 in a small volume of water and mixed it with a solution containing the enzyme PFOR, the unlabeled [12C]pyruvate and other cofactors. In the process, the use of Vitamin B12 was beneficial to protect the enzyme from the dissolved oxygen in water.39 After incubation at 37 °C for 15 min, the [11C]pyruvate was purified by sublimation with a 80 % RCY (Scheme 12).
The study of the metabolic pathways involved in the degradation of phenol have been extensively studied.40 The first step of this process was the carboxylation of phenol onto 4-hydroxybenzoate (72), through a Kolbe-Schmitt type reaction. In Pseudomonas, this step involved an enzyme know as phenol carboxylase, which has been exploited by Fuchs and co-workers to perform a CIE of 4-hydroxybenzoate with [14C]CO2 (Scheme 13).41, 42 Under optimal conditions, at pH 7 with 1 mM of 72, phosphate buffer, MnCl2 and dithioerythritol (DTE), an exchange rate of 100 nmol min−1 mg−1 cell protein was reported. Due to the specificity of the enzyme, the CIE was extended to only five related substrates, while the presence of the phenol in para position appeared to be essential.
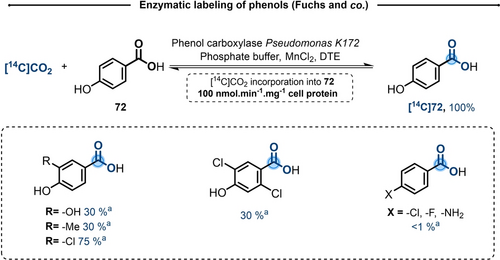
Enzymatic labeling of phenols using phenol carboxylase from Pseudomonas K172, DTE=dithioerythritol. a) Enzyme activity obtained with different aromatics compared to standard activity with 4-hydroxybenzoate (100 % represents 100 nmol [14C]CO2 incorporated into 4-hydroxybenzoate derivative. min−1 mg−1 cell protein).
2.5 Full Isotope Replacement
Isotope dilution is an obligation when equilibria and dynamic processes are involved in the exchange. To achieve high isotope incorporation (>90 IE), theoretically these transformations mandate using a large excess of isotopic source. For example, for an IE of 90 %, a minimum of 9 fold excess of the isotope source will be necessary.24 Such a large excess might be questionable, when expensive and radioactive sources are used. Consequently, methods aiming at late-stage Full Isotopic Replacement (FIR) have been developed.
2.5.1 Decarboxylative Cyanation
Isotope replacement of a carboxylic acid group is a strategy that has been explored for a long time through multi-step deconstruction/reconstruction strategy.43 An advancement was provided in the early 90s, when Barton reported the development of the photoinduced decarboxylative cyanation using sulfonyl cyanide as a nitrile source.44 However, the necessity of extra preparation steps (Barton ester synthesis and sulfonyl cyanide preparation) and the excess of nitrile source left this strategy completely unexplored for carbon labeling (Scheme 14). In contrast, the classical Barton radical decarboxylative halogenation was extensively used in radiolabeling.45
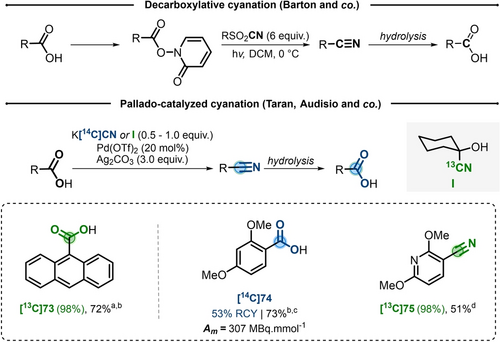
FIR using a decarboxylative cyanation step. a) Using I as nitrile source (1.0 equiv). b) After nitrile hydrolysis. c) Using K[13/14C]CN (0.5 equiv) as nitrile source and Pd(TFA)2 instead of Pd(OTf)2. d) Using K[13/14C]CN (0.25 equiv) and Pd(TFA)2 instead of Pd(OTf)2.
In 2010, Taran and co-workers developed a palladium-catalyzed decarboxylative cyanation suitable for carbon labeling.46 In presence of Pd(OTf)2 (20 mol %) and Ag2CO3 (3 equiv), benzoic acids were converted to the corresponding nitriles, using cyclohexanone cyanohydrin as cyanide source. The application of this strategy to 13C-labeling of 9-anthracenecarboxylic acid 73 afforded, after nitrile hydrolysis, its isotopologue [13C]73 in 72 % yield and 98 % IE (Scheme 14). In 2015, an improved version of this reaction with commercial K[14C]CN, in place of cyclohexanone cyanohydrin, was applied to the full isotopic exchange on 2,4-dimethoxybenzoic acid 74 (Scheme 14).47 Nonetheless, the scope of these reactions remains limited to ortho-substituted electron-rich carboxylic acids.
In 2017, Song and co-workers reported a complementary approach, based on the use 13C and 14C-labeled electrophilic cyanating agents.48 The use of N-cyano-N-phenyl p-toluenesulfonamide (NCTS) in the palladium-catalyzed decarboxylative cyanation of aryl carboxylic acids proved to be similar in scope. On the other hand, alkyl carboxylic acids underwent sequential α-cyanation/decarboxylation in presence of a strong base (LDA) and [13C]NCTS or [13C]1-H-benzotriazole-1-carbonitrile. Thus 2-(3,4-dichlorophenyl)acetic acid 76 and Naproxen 77 could be easily converted to the corresponding labeled nitriles (Scheme 15, top).
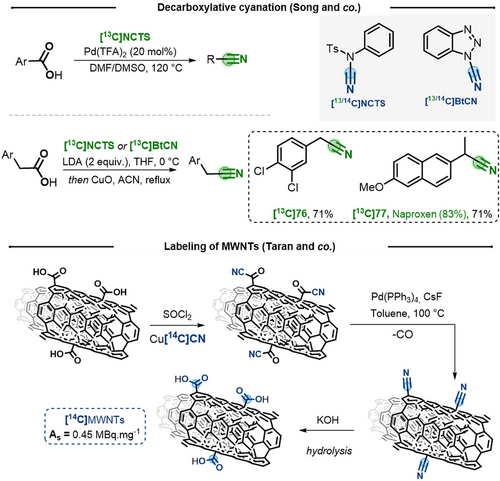
Top) Decarboxylative cyanation using electrophilic nitrile sources. Bottom) Labeling of MWNT by decarbonylation of aroyl cyanide.
Aiming to label Multi-Walled Carbon Nanotubes (MWNT) with 14C for further biodistribution studies, in 2010 Taran and co-workers explored another approach for FIR of carboxylic acids.49 Based on a palladium-mediated decarbonylation of aroyl cyanides, this strategy was optimized on 9-anthracene carboxylic acid, a substrate that best mimicked the aromatic network of MWNT (Scheme 15, bottom). After formation of the 13C-aroyl cyanide from Cu[13C]CN and the aroyl chloride, Pd(PPh3)4 catalyzed decarbonylation, in presence of CsF, to obtain the corresponding 13C-labeled nitrile. After hydrolysis, the label [13C]anthracene–9-carboxylic acid was obtained in 53 % overall yield and 98 % IE. This method allowed the first radio-labeling of [14C]MWNT with a specific activity (As) of 0.45 MBq mg−1 and their subsequent in vivo use to determine their biodistribution.
2.5.2 Decarboxylative Halogenation
While nitriles are well-embraced precursors of carboxylic acids, their hydrolysis might require harsh conditions, undermining the integrity of the molecule. In 2021, the group of Skrydstrup published a FIR involving a halogenated intermediate. The strategy was articulated around a nickel-mediated alkoxycarbonylation on alkyl iodides generated from the corresponding carboxylic acids.50 This three steps procedure has proven to be effective on the full labeling of 8 bioactive molecules with yields spanning from 31 % to 89 % (Scheme 16). Scope investigations showed compatibility of the method with sulfones, ketones, alkenes, carboxylic esters and azetidines.
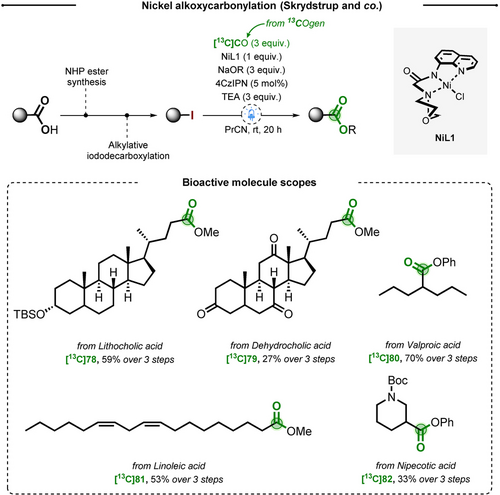
Nickel-catalyzed alkoxycarbonylation for FIR of carboxylic acids.
3 Carbon Isotope Exchange of Nitriles
Since 2018, CIE emerged as a viable alternative to multi-step radio-labeling, but the scope of this technology has been essentially limited to carboxylic acid derivatives. In order to overcome such restriction, the exchange of the C−CN bond was appealing, as the nitrile group is present in a remarkable number of biologically active compounds51 and, in addition, it provides a useful handle for further functionalization.52
3.1 Pioneering Examples of C−CN Bond Exchange
While modern examples of metal-catalyzed nitrile bond exchange have been rationalized and developed only very recently (see below), experimental observations of the reversible formation of the C−CN bond on specific substrates have been seldom reported in the literature. It is important to state that some of these historical observations have been controversial over the years. Nonetheless, we decided to dedicate them a section of this review.
To the best of our knowledge, the first observation of isotope exchange between alkyl nitriles and labeled cyanide salts was reported as early as 1949 by Martin D. Kamen,53 who nine years earlier discovered the long-lived 14C, in his seminal studies with Samuel Ruben.54 In this work, a moderate isotope exchange between β-hydroxypropionitrile and Na[14C]CN was reported under alkaline conditions (aqueous NaOH 0.1 M to 1 M) at 75 to 100 °C. Yet, this process appeared to be substrate specific, as no exchange was observed when such conditions were applied to acetonitrile, benzonitrile and succinonitrile.
Exchange between K[14C]CN and acetonitrile was published in 1980 by the group of Digenis.55 While synthesizing 14C-benzyl cyanide from the corresponding benzyl chloride, in presence of K[14C]CN and 18-crown-6 in acetonitrile, the authors observed a loss of the total radioactivity in the isolated product (Scheme 17, top). Unfortunately, the experimental details were not provided. When the authors reproduced the reaction with stable K[13C]CN, the reaction solvent (i.e. acetonitrile) was distilled and analyzed by NMR spectroscopy, seemingly showing 13C-enrichments. Care should be given on these results, as their significance was later questioned by the authors themselves.56 In addition, White and Chen opposed to such results and suggested that contaminations with H[14C]CN might have led to misinterpretation.57 However, they also stated that cyanide ion exchange with acetonitrile might occur to a minor extent under such reaction conditions. Indeed, it is conceivable that the extraordinarily higher sensitivity of β-emission from 14C, compared to NMR spectroscopy, could enable to detect even minimal exchange.
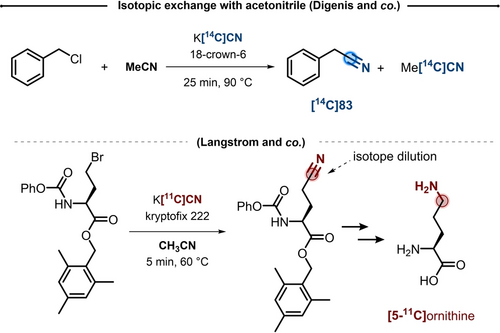
Isotopic dilution observed in presence of acetonitrile.
In 1989, the group of Langstrom provided further evidence on the role of acetonitrile in the isotopic dilution of 11C-labeled cyanide ions.58 In the course of their synthesis of [5-11C]ornithine, the 11C tracer was installed by nucleophilic substitution on protected 2-amino-4-bromobutanoic acid (Scheme 17, bottom) in presence of KOH, Kryptofix 222 and acetonitrile at 60 °C. The authors found that unlabeled cyanide ions were generated in acetonitrile, thus reducing the Am of the desired product. Interestingly, background reactions showed that the presence of KOH, Kryptofix 222 and acetonitrile were all required for the generation of the unlabeled cyanide ions.
In 1985, Digenis and co-workers reported a cyanide exchange on mandelonitrile (Scheme 18, top).59 This cyanohydrin was labeled in presence of a mixture of crown ethers in THF under thermal conditions, both using Na[13/14C]CN. To identify the product, [14C]mandelonitrile was reduced to phenylethanolamine [14C]85 (Scheme 18, A), which was thus obtained in 20 % RCY, based on the initial amount of Na[14C]CN (Scheme 18, A). It was speculated that exchange might occur upon equilibration between the cyanohydrin and benzaldehyde. To gain additional information, experiments were made with adamantane carbonitrile (86) and α-methoxyphenylacetonitrile (87). Interestingly, exchange was observed on highly hindered adamantane with 36 % RCY (Scheme 18, B) and labeled α-methoxyphenylacetonitrile ([14C]87) was isolated in 7 % RCY (Scheme 18, top). These results point towards a more complex reaction mechanism than the sole intermediacy of the corresponding aldehyde. However, substrate specific pathways cannot be excluded, due to the lack of additional investigations.

Nitrile isotope exchange on cyanohydrin. a) As reported by the authors in the original paper. b) Calculated after reduction of corresponding nitrile.
A notable application of this approach was reported in 1988 by Thomas and co-workers.60 In their studies on anticancer doxorubicin analogues, the authors aimed to label anthracyclines CM-DXR (88) and CM-DNR (89), bearing a peculiar cyanomorpholinyl substitution on the sugar moiety. The cyanide exchange strategy was attempted in presence of excess of isotopically diluted K[14C]CN (14 % IE) in aqueous conditions (Scheme 18, bottom). Labeled CM-DXR [14C]88 was isolated in 74 % yield (based on the anthracycline as limiting reagent), and a modest Am of 199 MBq mmol−1 (9 % IE). A similar outcome was observed with the structurally related CM-DNR [14C]89.
In 2011, Brewer and co-workers reported a nickel-catalyzed synthesis of isotopologues of the Unnatural Amino Acid (UAA) 4-cyano-L-phenylalanine (PheCN).61 This UAA has been previously incorporated into peptides and larger proteins, thus providing insight on the local environment of the proteins infrared and Raman spectroscopy.62 Starting from precursor 90, the author used a cyanation protocol reported by Kunz,63 with catalytic amounts of Ni(PPh3)2Br2, triphenylphosphine, zinc powder and K[12C]CN in acetonitrile. The desired Boc-PheCN-OMe (91) was isolated in 58 % yield. Next, the reaction was reproduced with the three isotopologues: KC[15N]N, K[13C]CN and K[13C,15N]CN (Scheme 19, top). Fourier Transformed Infrared Spectroscopy was chosen as analytical tool to determine the ratio of nitrile isotopologues, because of their specific vibrational profiles (with defined absorbance bands between 2229 and 2148 cm−1). Surprisingly, in all 13C and 15N-labeled products the IR spectra showed two absorbance bands: a major one corresponding to the desired labeled nitrile, and a minor band of the unlabeled nitrile (≈20 % by integration of the two bands). When the reaction time was increased to 24 h, an intensification of the signal of unlabeled nitrile was observed (up to 43 %), while full isotopic transfer (98 % 13C enrichment) from K[13C]CN was obtained with DMF as solvent. These experiments highlighted acetonitrile as a cause of isotopic dilution. Interestingly, while acetonitrile and related alkylnitriles have been shown as competent cyanide sources in transition-metal-catalyzed cyanations,64 here the absence of KCN prevented any product formation.
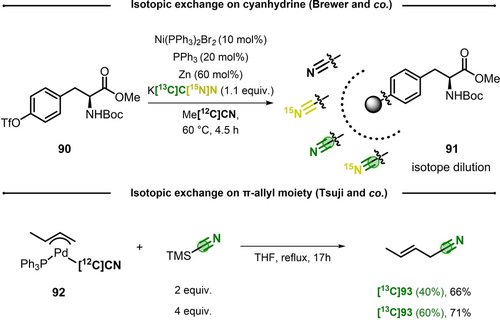
Top) CIE on UAA over nickel catalyzed cyanation. Bottom) CIE on palladium π-allyl complexes with TMS[13C]CN.
In their study on palladium-catalyzed cyanations of allylic carbonates, Tsuji et al. performed stoichiometric experiments to unveil the reaction mechanism.65 Interestingly, π-allyl complex (92) underwent nitrile isotope exchange with excess of TMS[13C]CN and (E)-2-butenyl cyanide 93 was isolated as mixture of isotopomers (Scheme 19, bottom). This suggested that the palladium cyanide bond was labile and exchange occurred prior to the reductive elimination step. In addition, IE was found directly proportional to the stoichiometry of TMS[13C]CN.
3.2 Nickel-Catalyzed CIE of Nitriles
While sporadic examples of nitrile isotope exchange have been essentially limited to experimental curiosity (see above), recently metal-catalyzed CIE of nitriles has emerged as an effective technology to label biologically active compounds. In early 2021, the groups of Audisio and Strotman independently reported the nickel-catalyzed CIE of aryl nitriles via a reversible decyanation.66, 67 Both publications utilized Zn([13C or 14C]CN)2 as isotopic source, under nickel catalysis. The choice of nickel to activate the challenging C(sp2)-CN bond (bond-dissociation energy≈130 kcal mol−1)68 was driven by the well-established literature precedents on this topic.69
On one hand, Audisio and co-workers laid out a catalytic system with Ni(COD)2, in presence of a trimethylphosphine (2.0 equiv), DMAP and 0.65 equivalents of Zn([13C]CN)2 in toluene. On another hand, Reilly et al. optimized two procedures based on the use of Lewis acids trimethylaluminium (AlMe3) or triphenylborane (BPh3) at 80 mol %, in NMP. In particular, BPh3 was most suitable for sensitive substrates due to the important reactivity of AlMe3. In either case, 1.2 equivalents of Zn[13C](CN)2 are employed.
Large functional group tolerance were shown in the two publications, with ortho, meta and para substituted aryl-nitriles successfully enriched with IE generally exceeding 40 % (Scheme 20). Numerous heterocyclic substrates were compatible, as well. For comparison, common substrates reported in the two articles are illustrated in Scheme 20 (bottom) and show the similarity of the methods. It is essential to bear in mind that, as consequence of the different stoichiometry in Zn[13C](CN)2 (0.65 equiv and 1.2 equiv), the maximal theoretical IE are 56 % (Audisio et al.) and 71 % (Strotman et al.), respectively. In both cases, the application to radiocarbon allowed to isolate the desired 14C-labeled pharmaceuticals. Stoichiometric experiments performed by Audisio's group allowed to isolate and characterize key nickel(II) intermediate (I, Scheme 20) obtained after oxidative addition. Furthermore, when Zn[13C](CN)2 was added to the complex, 12C/13C-cyanide isotope equilibration was monitored by 31P NMR. On the other hand, computational studies by Reilly et al. investigated the role of Lewis acids and found that it should not affect the oxidative addition or reductive elimination steps.70 Accordingly, the Lewis acid would interact after oxidative addition to favor cyanide abstraction,71, 72 by stabilizing the cyanide leaving group, thus facilitating CN exchange.
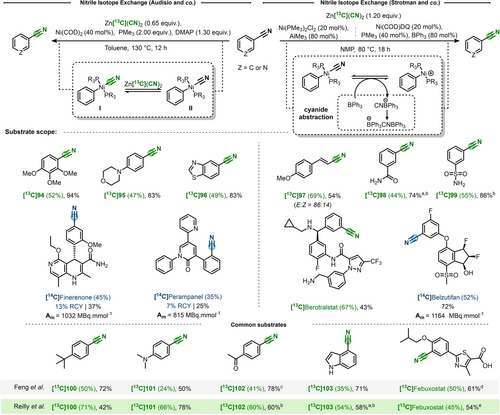
CIE on nitriles. a) 2 equiv of Lewis acid. b) PPh2Me instead of PMe3. c) 8 h. d) Performed on methyl ester. e) Ni(COD)DQ (40 mol %), PPh2Me (80 mol %), BPh3 (2.0 equiv) at 100 °C.
4 CIE on Other Functional Groups
Though recent breakthroughs in modern CIE on carboxylic acids and nitriles paved the way to more effective late-stage labeling, a large window of opportunity is still present to palliate current limitations. While these functional groups are popular, they rate at the 9th and 16th positions respectively, for the most frequent groups present in bioactive molecules.73 CIE is thus still in its infancy compared to HIE, and implementation of this strategy to other main chemical functions is still hardly needed. In the next section, we aim to provide a short overview on advances made in the field.
4.1 CIE of Terminal Alkenes
Alkenes are important moieties in organic synthesis and are frequently found in pharmaceutical compounds.74 As such, the development of late-stage labeling methodologies tackling this substructure is of great interest. Traditionally, terminal alkenes can be labeled by a two-step deconstruction/reconstruction strategy, allowing for an overall isotope exchange (Scheme 21). This is usually performed by (1) oxidative cleavage of the alkene, followed by a (2) Wittig olefination with a labeled phosphonium ylide. This approach has enabled labeling of Active Pharmaceutical Ingredient (API), such as [14C]β-Pinene,75 [14C]Lanosterol, [14C]Desmosterol,76 [14C]Gibberellin77 and [14C]Acanthoic acid78 (Scheme 21). Nonetheless, there are shortcomings to this procedure: a) the oxidative cleavage usually implies the utilizations of harsh conditions and hazardous chemicals (ozone, OsO4, …); b) despite some possibilities to discriminate electron rich and electron poor alkenes,79 oxidative cleavage could be highly challenging to perform on complex structures; c) the preparation of the 14C-labeled phosphonium ylide from [14C]CH3I, obviously entails multiple steps from [14C]CO2.
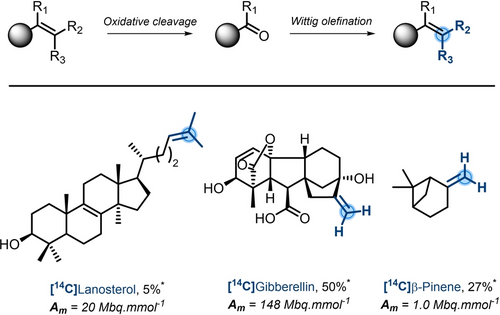
Oxidative cleavage/olefination strategy for the labeling of alkenes. *Overall chemical yield.
Based on the well-established alkene metathesis reaction,80 in 2004 Horstmann and Lewis patented its application to carbon isotope labeling.81 Taking advantage from the reversibility of this transformation, the authors showed proof-of-concept for CIE in presence of 13C and 14C-labeled ethylene. While, its implementation required a typical experimental set-up for [14C2]ethylene manipulation, the potential of the technology was showcased on ER-810951 (104), a challenging halichondrin analog (Scheme 22, top). Interestingly, full selectivity of the exchange on the less hindered alkene of the macrocycle was observed. Using 2nd generation Grubbs catalyst and single charges of 3 equiv of [14C2]ethylene (Am=4300 MBq mmol−1) under mild conditions, [14C]104 was obtained in 50 % IE. To increase the IE, left over isotopically diluted acetylene was evacuated, the 14C-product purified and engaged, three additional times, under the same reaction conditions. Overall, [14C]104 was isolated in 62 % yield, with a Am of 1800 MBq mmol−1, corresponding to 86 % IE. It should be noted that on the whole process 12 equivalents of radio-labeled ethylene were utilized.
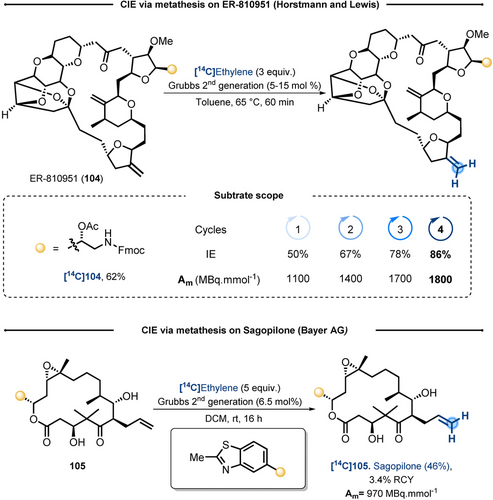
Application of metathesis with [14C2]ethylene to CIE on terminal alkene.
Another example of this methodology was performed on Sagopilone 105.82 While the detailed experimental procedure was not fully reported, the conditions are overall analogous to the precedent example: [14C2]ethylene (Am=2100 MBq mmol−1) was used in a 5 fold excess, dichloromethane in place of toluene and the reaction was performed at room temperature (Scheme 22, bottom). These conditions afforded [14C]105 Sagopilone in an Am of 970 MBq mmol−1 (42 % IE). Given the large excess of [14C2]ethylene, the RCY of this step was low (3.4 %), but the benefits of the direct labeling largely outperformed the lengthiness of more traditional radio-synthetic procedures.83
The major inconvenience of this strategy are the cost and the difficult accessibility to [14C2]ethylene,84 which highly prevented its practical utilization.85
4.2 Methyl Sulfone Labeling
Sulfones are recurrent functional groups in medicinal chemistry. In particular, methyl sulfones are found in a number of pharmaceuticals and even commercial drugs (Scheme 23, top).86
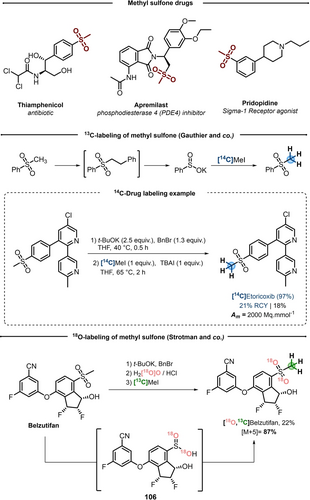
CIE performed on methyl sulfones.
In 2016, Gauthier and co-workers disclosed a synthesis of sulfinic acids starting from methyl sulfones.87 The strategy is articulated around a tandem benzylation/elimination in one-pot two-steps. To a solution of methyl sulfone and potassium tert-butoxide was added benzyl bromide, and after one hour at room temperature, the sulfinate salts could be easily filtered off. A dozen of sulfinic acids were thus obtained with good yields, showing good functional group tolerance, though a drop in yields was observed for alkyl methyl sulfones. Interestingly, this reaction was explored in the area of CIE, by simply trapping the in situ generated sulfinate salt with [14C]CH3I. Following this deconstruction/reconstitution technique, the authors attempted the 14C-labeling of API (Scheme 23, middle). Standard conditions were suitable for Etoricoxib, despite competitive pyridine N-methylations during the second step. In spite of moderate yield also due to technical issues in the transfer of volatile, radioactive [14C]CH3I, [14C]Etoricoxib was isolated in 18 % overall yield (Am of 2000 MBq mmol−1).
Based on this methodology, in 2020, Strotman and co-workers developed a sequential strategy for stable isotope labeling of Belzutifan, with 18O and 13C.88 After filtration, sulfinate 106 was dissolved in H2[18O]O followed by addition of HCl (1.5 equiv). After 60 minutes at 80 °C, the reaction mixture was neutralized, [13C]CH3I (3 equiv) was added and the reaction was ran over 3 hours affording [18O2, 13C]Belzutifan in 22 % overall yield and 87 % [M+5] enrichment due to the presence multiple stable isotopes on this scaffold.
5 Conclusion and Outlook
After decades of low-key innovation, the field of CIL has recently explored new alternative avenues. The hurdles of radiochemistry with long-lived isotopes and skyrocketing prices of starting materials have forced chemists to explore complementary retrosynthetic approaches, which can be implemented at a late-stage of the process. An emerging opportunity was provided by the recently coined CIE technology. Defined as the selective replacement of a 12C-functional group (i.e. −COOH, −CN) with its radiolabeled counterpart in one single operation, CIE aims to explore reversible C−C bond cleavage strategies using easily accessible C-labeled building blocks. The aim of this review is to highlight the range of creative and innovative CIE methodologies that have been explored, and particularly in the relatively short timeframe since the emergence in 2018 of modern CIE, within the (radio)synthetic community. Although important progresses were witnessed with CIE of carboxylic acids and nitrile derivatives, considerable room for improvement still exists. For some of the methods, the scope is limited to specific scaffolds such as phenyl acetic acids and the use of high excess of labeled precursor is unsatisfactory. Notably the implementation of enantioselective variants still remains a visionary goal. In light of the recent developments and inherent limitations, this emerging field of research provides further avenues for future explorations.
Acknowledgments
This project was supported by the European Union's Horizon 2020 research innovation program FET-OPEN No 862179 and the European Research Council (ERC-2019-COG—864576). We thank the CEA of Saclay for constant support of our research activities. The authors warmly thank Clara Zavarise (DRF-JOLIOT-SCBM, CEA) for the cover artwork and Rémi Blieck for proof-reading.
Conflict of interest
The authors declare no conflict of interest.
Biographical Information
Alexandre Labiche received a master degree specialized in biomolecules and organic synthesis in 2020 at the university of Rouen (France). Since, he started a PhD position at CEA (Paris-Saclay, France) directed by Dr. Davide Audisio in the “Molecular Labeling and Bio-Organic Chemistry” laboratory, led by Dr. Frederic Taran. His research topic is the development of new methodologies giving access to radiolabeled organic compounds, using carbon isotopes.
Biographical Information
Augustin Malandain obtained his M.Sc. in chemistry in 2021 at the national graduate school of chemistry of Montpellier (France). Thereafter he started his PhD in organic chemistry/radiolabeling at Atomic Energy Commission CEA (France) under the supervision of Dr. Davide Audisio. His scientific interests focus on the development of new late-stage carbon labeling methodologies.
Biographical Information
Maxime Molins received his M.Sc in chemistry and life sciences in 2021 at the PSL university (Paris, France). Since, he has been a PhD student in Dr. Davide Audisio's group, at Atomic Energy Commission CEA (France). His research focus on the development of new carbon isotope exchange methodologies for Carbon 14 radiolabeling.
Biographical Information
Frédéric Taran secured a PhD in chemistry at the Paris XI University under the supervision of Dr. Charles Mioskowski and then moved to a post-doctoral position with Prof. Sir Derek Barton at Texas A&M University. Since 1998, he works for the French Alternative Energies and Atomic Energy Commission (CEA) located at Saclay, close to Paris and is now in charge of the department of bioorganic chemistry.
Biographical Information
Davide Audisio is a group leader at the Atomic Energy Commission CEA (France) since 2016. His research interests include carbon isotope labeling and isotope exchange, the development of bio-orthogonal chemistry tools and alternative synthetic access to poly-aromatics. He is recipient of an ERC Consolidator Grant, Jean-Pierre Sauvage Award 2021 and elected member of the French Academy of Pharmacy in 2021.