Graphene Mediates Charge Transfer between Lead Chromate and a Cobalt Cubane Cocatalyst for Photocatalytic Water Oxidation
Wenchao Jiang
School of Chemistry and Materials Science, University of Science and Technology of China, 230026 Hefei, China
State Key Laboratory of Catalysis, Dalian National Laboratory for Clean Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 116023 Dalian, China
Contribution: Data curation (lead), Formal analysis (lead), Investigation (lead), Writing - original draft (lead), Writing - review & editing (lead)
Search for more papers by this authorLingcong Zhang
State Key Laboratory of Catalysis, Dalian National Laboratory for Clean Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 116023 Dalian, China
University of Chinese Academy of Sciences, Center of Materials Science and Optoelectronics Engineering, 100049 Beijing, China
Contribution: Formal analysis (supporting)
Search for more papers by this authorChenwei Ni
State Key Laboratory of Catalysis, Dalian National Laboratory for Clean Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 116023 Dalian, China
University of Chinese Academy of Sciences, Center of Materials Science and Optoelectronics Engineering, 100049 Beijing, China
Contribution: Formal analysis (supporting)
Search for more papers by this authorMing Shi
State Key Laboratory of Catalysis, Dalian National Laboratory for Clean Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 116023 Dalian, China
University of Chinese Academy of Sciences, Center of Materials Science and Optoelectronics Engineering, 100049 Beijing, China
Contribution: Formal analysis (supporting)
Search for more papers by this authorYue Zhao
State Key Laboratory of Catalysis, Dalian National Laboratory for Clean Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 116023 Dalian, China
University of Chinese Academy of Sciences, Center of Materials Science and Optoelectronics Engineering, 100049 Beijing, China
Contribution: Formal analysis (supporting)
Search for more papers by this authorYuting Deng
State Key Laboratory of Catalysis, Dalian National Laboratory for Clean Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 116023 Dalian, China
University of Chinese Academy of Sciences, Center of Materials Science and Optoelectronics Engineering, 100049 Beijing, China
Contribution: Formal analysis (supporting)
Search for more papers by this authorHaibo Chi
School of Chemistry and Materials Science, University of Science and Technology of China, 230026 Hefei, China
State Key Laboratory of Catalysis, Dalian National Laboratory for Clean Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 116023 Dalian, China
Contribution: Formal analysis (supporting)
Search for more papers by this authorRuotian Chen
State Key Laboratory of Catalysis, Dalian National Laboratory for Clean Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 116023 Dalian, China
University of Chinese Academy of Sciences, Center of Materials Science and Optoelectronics Engineering, 100049 Beijing, China
Contribution: Formal analysis (supporting)
Search for more papers by this authorProf. Xiuli Wang
State Key Laboratory of Catalysis, Dalian National Laboratory for Clean Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 116023 Dalian, China
University of Chinese Academy of Sciences, Center of Materials Science and Optoelectronics Engineering, 100049 Beijing, China
Contribution: Formal analysis (supporting)
Search for more papers by this authorCorresponding Author
Prof. Rengui Li
State Key Laboratory of Catalysis, Dalian National Laboratory for Clean Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 116023 Dalian, China
University of Chinese Academy of Sciences, Center of Materials Science and Optoelectronics Engineering, 100049 Beijing, China
Contribution: Formal analysis (lead), Funding acquisition (lead), Investigation (equal), Writing - original draft (lead), Writing - review & editing (lead)
Search for more papers by this authorCorresponding Author
Prof. Can Li
School of Chemistry and Materials Science, University of Science and Technology of China, 230026 Hefei, China
State Key Laboratory of Catalysis, Dalian National Laboratory for Clean Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 116023 Dalian, China
Search for more papers by this authorWenchao Jiang
School of Chemistry and Materials Science, University of Science and Technology of China, 230026 Hefei, China
State Key Laboratory of Catalysis, Dalian National Laboratory for Clean Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 116023 Dalian, China
Contribution: Data curation (lead), Formal analysis (lead), Investigation (lead), Writing - original draft (lead), Writing - review & editing (lead)
Search for more papers by this authorLingcong Zhang
State Key Laboratory of Catalysis, Dalian National Laboratory for Clean Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 116023 Dalian, China
University of Chinese Academy of Sciences, Center of Materials Science and Optoelectronics Engineering, 100049 Beijing, China
Contribution: Formal analysis (supporting)
Search for more papers by this authorChenwei Ni
State Key Laboratory of Catalysis, Dalian National Laboratory for Clean Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 116023 Dalian, China
University of Chinese Academy of Sciences, Center of Materials Science and Optoelectronics Engineering, 100049 Beijing, China
Contribution: Formal analysis (supporting)
Search for more papers by this authorMing Shi
State Key Laboratory of Catalysis, Dalian National Laboratory for Clean Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 116023 Dalian, China
University of Chinese Academy of Sciences, Center of Materials Science and Optoelectronics Engineering, 100049 Beijing, China
Contribution: Formal analysis (supporting)
Search for more papers by this authorYue Zhao
State Key Laboratory of Catalysis, Dalian National Laboratory for Clean Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 116023 Dalian, China
University of Chinese Academy of Sciences, Center of Materials Science and Optoelectronics Engineering, 100049 Beijing, China
Contribution: Formal analysis (supporting)
Search for more papers by this authorYuting Deng
State Key Laboratory of Catalysis, Dalian National Laboratory for Clean Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 116023 Dalian, China
University of Chinese Academy of Sciences, Center of Materials Science and Optoelectronics Engineering, 100049 Beijing, China
Contribution: Formal analysis (supporting)
Search for more papers by this authorHaibo Chi
School of Chemistry and Materials Science, University of Science and Technology of China, 230026 Hefei, China
State Key Laboratory of Catalysis, Dalian National Laboratory for Clean Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 116023 Dalian, China
Contribution: Formal analysis (supporting)
Search for more papers by this authorRuotian Chen
State Key Laboratory of Catalysis, Dalian National Laboratory for Clean Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 116023 Dalian, China
University of Chinese Academy of Sciences, Center of Materials Science and Optoelectronics Engineering, 100049 Beijing, China
Contribution: Formal analysis (supporting)
Search for more papers by this authorProf. Xiuli Wang
State Key Laboratory of Catalysis, Dalian National Laboratory for Clean Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 116023 Dalian, China
University of Chinese Academy of Sciences, Center of Materials Science and Optoelectronics Engineering, 100049 Beijing, China
Contribution: Formal analysis (supporting)
Search for more papers by this authorCorresponding Author
Prof. Rengui Li
State Key Laboratory of Catalysis, Dalian National Laboratory for Clean Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 116023 Dalian, China
University of Chinese Academy of Sciences, Center of Materials Science and Optoelectronics Engineering, 100049 Beijing, China
Contribution: Formal analysis (lead), Funding acquisition (lead), Investigation (equal), Writing - original draft (lead), Writing - review & editing (lead)
Search for more papers by this authorCorresponding Author
Prof. Can Li
School of Chemistry and Materials Science, University of Science and Technology of China, 230026 Hefei, China
State Key Laboratory of Catalysis, Dalian National Laboratory for Clean Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 116023 Dalian, China
Search for more papers by this authorGraphical Abstract
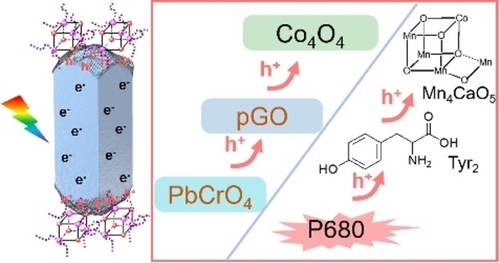
Partially oxidized graphene (pGO) operates as a charge-transfer mediator between the water oxidation cocatalyst (Co4O4) and the hole-accumulating {−101} facets of PbCrO4. Unimpeded transfer of photogenerated holes from PbCrO4 to Co4O4 via the pGO mediator is demonstrated. The resulting Co4O4/pGO/PbCrO4 photocatalyst oxidizes water with an apparent quantum efficiency exceeding 10 % at 500 nm.
Abstract
The interfacial barrier of charge transfer from semiconductors to cocatalysts means that the photogenerated charges cannot be fully utilized, especially for the challenging water oxidation reaction. Using cobalt cubane molecules (Co4O4) as water oxidation cocatalysts, we rationally assembled partially oxidized graphene (pGO), acting as a charge-transfer mediator, on the hole-accumulating {−101} facets of lead chromate (PbCrO4) crystal. The assembled pGO enables preferable immobilization of Co4O4 molecules on the {−101} facets of the PbCrO4 crystal, which is favorable for the photogenerated holes transferring from PbCrO4 to Co4O4 molecules. The surface charge-transfer efficiency of PbCrO4 was boosted by selective assembly of pGO between PbCrO4 and Co4O4 molecules. An apparent quantum efficiency for photocatalytic water oxidation on the Co4O4/pGO/PbCrO4 photocatalyst exceeded 10 % at 500 nm. This strategy of rationally assembling charge-transfer mediator provides a feasible method for acceleration of charge transfer and utilization in semiconductor photocatalysis.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202302575-sup-0001-misc_information.pdf1.4 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. Fujishima, K. Honda, Nature 1972, 238, 37–38;
- 1bN. S. Lewis, D. G. Nocera, Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735;
- 1cY. Tachibana, L. Vayssieres, J. R. Durrant, Nat. Photonics 2012, 6, 511–518;
- 1dM. G. Walter, E. L. Warren, J. R. McKone, S. W. Boettcher, Q. Mi, E. A. Santori, N. S. Lewis, Chem. Rev. 2010, 110, 6446–6473.
- 2K. J. Young, L. A. Martini, R. L. Milot, R. C. SnoebergerIII, V. S. Batista, C. A. Schmuttenmaer, R. H. Crabtree, G. W. Brudvig, Coord. Chem. Rev. 2012, 256, 2503–2520.
- 3
- 3aS. Berardi, G. G. La, M. Natali, I. Bazzan, F. Puntoriero, A. Sartorel, F. Scandola, S. Campagna, M. Bonchio, J. Am. Chem. Soc. 2012, 134, 11104–11107;
- 3bY. Wang, F. Li, X. Zhou, F. Yu, J. Du, L. Bai, L. Sun, Angew. Chem. Int. Ed. 2017, 56, 6911–6915.
- 4
- 4aY. Wang, F. Li, H. Li, L. Bai, L. Sun, Chem. Commun. 2016, 52, 3050–3053;
- 4bS. Ye, R. Chen, Y. Xu, F. Fan, P. Du, F. Zhang, X. Zong, T. Chen, Y. Qi, P. Chen, Z. Chen, C. Li, J. Catal. 2016, 338, 168–173.
- 5
- 5aJ. Barber, Chem. Soc. Rev. 2009, 38, 185–196;
- 5bJ. Yano, V. Yachandra, Chem. Rev. 2014, 114, 4175–4205.
- 6H. Dau, I. Zaharieva, Acc. Chem. Res. 2009, 42, 1861–1870.
- 7J. M. Keough, A. N. Zuniga, D. L. Jenson, B. A. Barry, J. Phys. Chem. B 2013, 117, 1296–1307.
- 8
- 8aY. Zhao, R. Li, L. Mu, C. Li, Cryst. Growth Des. 2017, 17, 2923–2928;
- 8bR. Li, X. Tao, R. Chen, F. Fan, C. Li, Chem. Eur. J. 2015, 21, 14337–14341;
- 8cY. J. Jeong, D. H. Seo, J. H. Baek, M. J. Kang, B. N. Kim, S. K. Kim, X. Zheng, I. S. Cho, Adv. Funct. Mater. 2022, 32, 2208196;
- 8dH. S. Han, S. Shin, D. H. Kim, I. J. Park, J. S. Kim, P.-S. Huang, J.-K. Lee, I. S. Cho, X. Zheng, Energy Environ. Sci. 2018, 11, 1299–1306;
- 8eY. J. Jeong, S. W. Hwang, S. Chaikasetsin, H. S. Han, I. S. Cho, Chem. Eng. J. 2022, 435, 135183.
- 9
- 9aR. Li, F. Zhang, D. Wang, J. Yang, M. Li, J. Zhu, X. Zhou, H. Han, C. Li, Nat. Commun. 2013, 4, 1432;
- 9bL. Mu, Y. Zhao, A. Li, S. Wang, Z. Wang, J. Yang, Y. Wang, T. Liu, R. Chen, J. Zhu, F. Fan, R. Li, C. Li, Energy Environ. Sci. 2016, 9, 2463–2469;
- 9cH. G. Yang, C. H. Sun, S. Z. Qiao, J. Zou, G. Liu, S. C. Smith, H. M. Cheng, G. Q. Lu, Nature 2008, 453, 638–641.
- 10
- 10aR. Li, H. Han, F. Zhang, D. Wang, C. Li, Energy Environ. Sci. 2014, 7, 1369–1376;
- 10bT. Takata, J. Jiang, Y. Sakata, M. Nakabayashi, N. Shibata, V. Nandal, K. Seki, T. Hisatomi, K. Domen, Nature 2020, 581, 411–414.
- 11W. Jiang, C. Ni, L. Zhang, M. Shi, J. Qu, H. Zhou, C. Zhang, R. Chen, X. Wang, C. Li, R. Li, Angew. Chem. Int. Ed. 2022, 61, e202207161.
- 12H. Kato, K. Asakura, A. Kudo, J. Am. Chem. Soc. 2003, 125, 3082–3089.
- 13S. Ye, C. Ding, R. Chen, F. Fan, P. Fu, H. Yin, X. Wang, Z. Wang, P. Du, C. Li, J. Am. Chem. Soc. 2018, 140, 3250–3256.
- 14S. Liu, J. Pan, W. Kong, X. Li, J. Zhang, X. Zhang, R. Liu, Y. Li, Y. Zhao, D. Wang, J. Zhang, S. Zhu, ACS Appl. Mater. Interfaces 2022, 14, 12180–12192.
- 15F. Bejarano, I. J. O. Contreras, A. Droghetti, I. Rungger, A. Rudnev, D. Gutiérrez, M. M. Torrent, J. Veciana, H. S. J. Zant, C. Rovira, E. Burzurí, N. Crivillers, J. Am. Chem. Soc. 2018, 140, 1691–1696.
- 16W. Jiang, X. Yang, F. Li, Q. Zhang, S. Li, H. Tong, Y. Jiang, L. Xia, Chem. Commun. 2019, 55, 1414–1417.
- 17D. K. Zhong, S. Choi, D. R. Gamelin, J. Am. Chem. Soc. 2011, 133, 18370–18377.
- 18
- 18aH. Wang, Y. Xia, X. Wang, Y. Han, X. Jiao, D. Chen, ACS Appl. Mater. Interfaces 2019, 11, 33062–33073;
- 18bP. Ghosh, A. Kar, S. Khandelwal, D. Vyas, A. Q. Mir, A. L. Chakraborty, R. S. Hegde, S. Sharma, A. Dutta, S. Khatua, ACS Appl. Nano Mater. 2019, 2, 5795–5803.
- 19T.-F. Yeh, C.-Y. Teng, S.-J. Chen, H. Teng, Adv. Mater. 2014, 26, 3297–3303.
- 20
- 20aR. Chen, F. Fan, C. Li, Angew. Chem. Int. Ed. 2022, 61, e202117567;
- 20bJ. Zhu, F. Fan, R. Chen, H. An, Z. Feng, C. Li, Angew. Chem. Int. Ed. 2015, 54, 9111–9114.