Superprotonic Conductivity of MOFs Confining Zwitterionic Sulfamic Acid as Proton Source and Conducting Medium
Dr. Amitosh Sharma
Department of Chemistry, Ulsan National Institute of Science and Technology (UNIST), Ulsan, 44919 Republic of Korea
These authors contributed equally to this work.
Search for more papers by this authorDr. Jaewoong Lim
Department of Chemistry, Ulsan National Institute of Science and Technology (UNIST), Ulsan, 44919 Republic of Korea
These authors contributed equally to this work.
Search for more papers by this authorSeonghwan Lee
Department of Chemistry, Ulsan National Institute of Science and Technology (UNIST), Ulsan, 44919 Republic of Korea
Search for more papers by this authorDr. Seungwan Han
Department of Chemistry, Ulsan National Institute of Science and Technology (UNIST), Ulsan, 44919 Republic of Korea
Search for more papers by this authorDr. Junmo Seong
Department of Chemistry, Ulsan National Institute of Science and Technology (UNIST), Ulsan, 44919 Republic of Korea
Search for more papers by this authorProf. Dr. Seung Bin Baek
Department of Chemistry, Ulsan National Institute of Science and Technology (UNIST), Ulsan, 44919 Republic of Korea
Search for more papers by this authorCorresponding Author
Prof. Dr. Myoung Soo Lah
Department of Chemistry, Ulsan National Institute of Science and Technology (UNIST), Ulsan, 44919 Republic of Korea
Search for more papers by this authorDr. Amitosh Sharma
Department of Chemistry, Ulsan National Institute of Science and Technology (UNIST), Ulsan, 44919 Republic of Korea
These authors contributed equally to this work.
Search for more papers by this authorDr. Jaewoong Lim
Department of Chemistry, Ulsan National Institute of Science and Technology (UNIST), Ulsan, 44919 Republic of Korea
These authors contributed equally to this work.
Search for more papers by this authorSeonghwan Lee
Department of Chemistry, Ulsan National Institute of Science and Technology (UNIST), Ulsan, 44919 Republic of Korea
Search for more papers by this authorDr. Seungwan Han
Department of Chemistry, Ulsan National Institute of Science and Technology (UNIST), Ulsan, 44919 Republic of Korea
Search for more papers by this authorDr. Junmo Seong
Department of Chemistry, Ulsan National Institute of Science and Technology (UNIST), Ulsan, 44919 Republic of Korea
Search for more papers by this authorProf. Dr. Seung Bin Baek
Department of Chemistry, Ulsan National Institute of Science and Technology (UNIST), Ulsan, 44919 Republic of Korea
Search for more papers by this authorCorresponding Author
Prof. Dr. Myoung Soo Lah
Department of Chemistry, Ulsan National Institute of Science and Technology (UNIST), Ulsan, 44919 Republic of Korea
Search for more papers by this authorGraphical Abstract
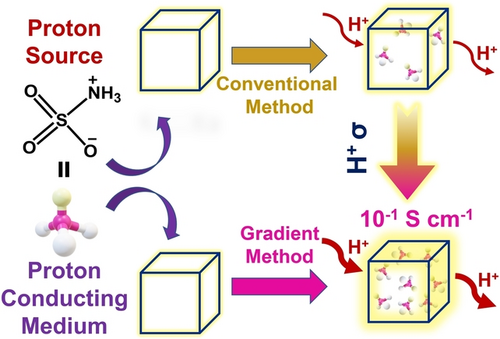
Strategic loading of dual-functioning zwitterionic sulfamic acid in MOFs leads to proton conductivity of the order of 10−1 S cm−1. This high conductivity is the result of higher loading of sulfamic acid, which can act as both a proton source due to its acidity and a conducting medium due to its extensive hydrogen-bonding ability and zwitterion effect.
Abstract
A few metal–organic frameworks (MOFs), which typically use strong acids as proton sources, display superprotonic conductivity (≈10−1 S cm−1); however, they are rare due to the instability of MOFs in highly acidic conditions. For the first time, we report superprotonic conductivity using a moderately acidic guest, zwitterionic sulfamic acid (HSA), which is encapsulated in MOF-808 and MIL-101. HSA acts not only as a proton source but also as a proton-conducting medium due to its extensive hydrogen bonding ability and zwitterion effect. A new sustained concentration gradient method results in higher HSA encapsulation compared to conventional methods, producing 10HSA@MOF-808-(bSA)2 and 8HSA@MIL-101. These MOFs show impressive superprotonic conductivity of 2.47×10−1 and 3.06×10−1 S cm−1, respectively, at 85 °C and 98 % relative humidity, and maintain stability for 7 days.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202302376-sup-0001-misc_information.pdf12.8 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1K. Jiao, J. Xuan, Q. Du, Z. Bao, B. Xie, B. Wang, Y. Zhao, L. Fan, H. Wang, Z. Hou, S. Huo, N. P. Brandon, Y. Yin, M. D. Guiver, Nature 2021, 595, 361–369.
- 2P. Costamagna, S. Srinivasan, J. Power Sources 2001, 102, 242–252.
- 3D. A. Cullen, K. C. Neyerlin, R. K. Ahluwalia, R. Mukundan, K. L. More, R. L. Borup, A. Z. Weber, D. J. Myres, A. Kusoglu, Nat. Energy 2021, 6, 462–474.
- 4L. Fan, Z. Tu, S. H. Chan, Energy Rep. 2021, 7, 8421–8446.
- 5M. Sadakiyo, T. Yamada, H. Kitagawa, J. Am. Chem. Soc. 2009, 131, 9906–9907.
- 6J. M. Taylor, R. K. Mah, I. L. Moudrakovski, C. I. Ratcliffe, R. Vaidhyanathan, G. K. H. Shimizu, J. Am. Chem. Soc. 2010, 132, 14055–14057.
- 7V. Guillerm, D. Kim, J. F. Eubank, R. Luebke, X. Liu, K. Adil, M. S. Lah, M. Eddaoudi, Chem. Soc. Rev. 2014, 43, 6141–6172.
- 8L. Heinke, C. Woll, Adv. Mater. 2019, 31, 1806324.
- 9W. Lu, Z. Wei, Z.-Y. Gu, T.-F. Liu, J. Park, J. Park, J. Tian, Q. Zhang, T. Gentle III, M. Bosch, H.-C. Zhou, Chem. Soc. Rev. 2014, 43, 5561–5593.
- 10Z. Ji, H. Wang, S. Canossa, S. Wuttke, O. M. Yaghi, Adv. Funct. Mater. 2020, 30, 2000238.
- 11A.-L. Li, Q. Gao, J. Xu, X.-H. Bu, Coord. Chem. Rev. 2017, 344, 54–82.
- 12Y. Ye, L. Gong, S. Xiang, Z. Zhang, B. Chen, Adv. Mater. 2020, 32, 1907090.
- 13P. Ramaswamy, N. E. Wong, G. K. H. Shimizu, Chem. Soc. Rev. 2014, 43, 5913–5932.
- 14K.-D. Kreuer, Chem. Mater. 1996, 8, 610–641.
- 15W. Kühlbrandt, Nature 2000, 406, 569–570.
- 16M. Rini, B.-Z. Magnes, E. Pines, E. T. J. Nibbering, Science 2003, 301, 349–352.
- 17D.-W. Lim, H. Kitagawa, Chem. Rev. 2020, 120, 8416–8467.
- 18K. Otake, H. Kitagawa, Small 2021, 17, 2006189.
- 19M. Sadakiyo, T. Yamada, K. Honda, H. Matsui, H. Kitagawa, J. Am. Chem. Soc. 2014, 136, 7701–7707.
- 20A. Sharma, J. Lim, M. S. Lah, Coord. Chem. Rev. 2023, 479, 214995.
- 21D.-W. Lim, H. Kitagawa, Chem. Soc. Rev. 2021, 50, 6349–6368.
- 22D. W. Kang, M. Kang, C. S. Hong, J. Mater. Chem. A 2020, 8, 7474–7494.
- 23V. G. Ponomareva, K. A. Kovalenko, A. P. Chupakhin, D. N. Dybtsev, E. S. Shutova, V. P. Fedin, J. Am. Chem. Soc. 2012, 134, 15640–15643.
- 24W. J. Phang, H. Jo, W. R. Lee, J. W. Song, K. Yoo, B.-S. Kim, C. S. Hong, Angew. Chem. Int. Ed. 2015, 54, 5142–5146.
- 25S. Bureekaew, S. Horike, M. Higuchi, M. Mizuno, T. Kawamura, D. Tanaka, N. Yanai, S. Kitagawa, Nat. Mater. 2009, 8, 831–836.
- 26D. Umeyama, S. Horike, M. Inuaki, Y. Hijikata, S. Kitagawa, Angew. Chem. Int. Ed. 2011, 50, 11706–11709.
- 27J. A. Hurd, R. Vaidhyanathan, V. Thangadurai, C. I. Ratcliffe, I. L. Moudrakovski, G. K. H. Shimizu, Nat. Chem. 2009, 1, 705–710.
- 28W.-L. Xue, W.-H. Deng, H. Chen, R.-H. Liu, J. M. Taylor, Y.-K. Li, L. Wang, Y.-H. Deng, W.-H. Li, Y.-Y. Wen, G.-E. Wang, C.-Q. Wan, G. Xu, Angew. Chem. Int. Ed. 2021, 60, 1290–1297.
- 29X.-M. Li, L.-Z. Dong, S.-L. Li, G. Xu, J. Liu, F.-M. Zhang, L.-S. Lu, Y.-Q. Lan, ACS Energy Lett. 2017, 2, 2313–2318.
- 30S. Devautour-Vinot, E. S. Sanil, A. Geneste, V. Ortiz, P. G. Yot, J.-S. Chang, G. Maurin, Chem. Asian J. 2019, 14, 3561–3565.
- 31F. Yang, G. Xu, Y. Dou, B. Wang, H. Zhang, H. Wu, W. Zhou, J.-R. Li, B. Chen, Nat. Energy 2017, 2, 877–883.
- 32S.-S. Liu, Z. Han, J.-S. Yang, S.-Z. Huang, X.-Y. Dong, S.-Q. Zang, Inorg. Chem. 2020, 59, 396–402.
- 33S. Mukhopadhyay, J. Debgupta, C. Singh, R. Sarkar, O. Basu, S. K. Das, ACS Appl. Mater. Interfaces 2019, 11, 13423–13432.
- 34Z.-H. Li, H. Zeng, G. Zeng, C. Ru, G. Li, W. Yan, Z. Shi, S. Feng, Angew. Chem. Int. Ed. 2021, 60, 26577–26581.
- 35X.-M. Li, J. Liu, C. Zhao, J.-L. Zhou, L. Zhao, S.-L. Li, Y.-Q. Lan, J. Mater. Chem. A 2019, 7, 25165–25171.
- 36S. Kim, B. Joarder, J. A. Hurd, J. Zhang, K. W. Dawson, B. S. Gelfand, N. E. Wong, G. K. H. Shimizu, J. Am. Chem. Soc. 2018, 140, 1077–1082.
- 37X.-M. Li, Y. Wang, Y. Mu, J. Liu, L. Zeng, Y.-Q. Lan, ACS Appl. Mater. Interfaces 2022, 14, 9264–9271.
- 38A. J. Howarth, Y. Liu, P. Li, Z. Li, T. C. Wang, J. T. Hupp, O. K. Farha, Nat. Rev. Mater. 2016, 1, 15018.
- 39S. Yuan, L. Feng, K. Wang, J. Pang, M. Bosch, C. Lollar, Y. Sun, J. Qin, X. Yang, P. Zhang, Q. Wang, L. Zou, Y. Zhang, L. Zhang, Y. Fang, J. Li, H.-C. Zhou, Adv. Mater. 2018, 30, 1704303.
- 40D. N. Dybtsev, V. G. Ponomareva, S. B. Aliev, A. P. Chupakhin, M. R. Gallyamov, N. K. Moroz, B. A. Kolesov, K. A. Kovalenko, E. S. Shutova, V. P. Fedin, ACS Appl. Mater. Interfaces 2014, 6, 5161–5167.
- 41H.-B. Luo, Q. Ren, P. Wang, J. Zhang, L. Wang, X.-M. Ren, ACS Appl. Mater. Interfaces 2019, 11, 9164–9171.
- 42F.-M. Zhang, L.-Z. Dong, J.-S. Qin, W. Guan, J. Liu, S.-L. Li, M. Lu, Z.-M. Su, H.-C. Zhou, J. Am. Chem. Soc. 2017, 139, 6183–6189.
- 43Y. Ye, W. Guo, L. Wang, Z. Li, Z. Song, J. Chen, Z. Zhang, S. Xiang, B. Chen, J. Am. Chem. Soc. 2017, 139, 15604–15607.
- 44M. V. Nguyen, T. H. N. Lo, L. C. Luu, H. T. T. Nguyen, T. T. Tu, J. Mater. Chem. A 2018, 6, 1816–1821.
- 45S. S. Nagarkar, S. M. Unni, A. Sharma, S. Kurungot, S. K. Ghosh, Angew. Chem. Int. Ed. 2014, 53, 2638–2642.
- 46R. L. Sass, Acta Crystallogr. 1960, 13, 320–324.
- 47Y.-S. Wei, X.-P. Hu, Z. Han, X.-Y. Dong, S.-Q. Zang, T. C. W. Mak, J. Am. Chem. Soc. 2017, 139, 3505–3512.
- 48M. Meilikhov, K. Yusenko, D. Esken, S. Turner, G. Van Tendeloo, R. A. Fischer, Eur. J. Inorg. Chem. 2010, 3701–3714.
- 49J. Juan-Alcañiz, J. Gascon, F. J. Kapteijn, Mater. Chem. 2012, 22, 10102–10118.
- 50H. Furukawa, F. Gándara, Y.-B. Zhang, J. Jiang, W. L. Queen, R. M. Hudson, O. M. Yaghi, J. Am. Chem. Soc. 2014, 136, 4369–4381.
- 51A. Sharma, J. Lim, J. S. Jeong, S. Won, J. Seong, S. Lee, Y. S. Kim, S. B. Baek, M. S. Lah, Angew. Chem. Int. Ed. 2021, 60, 14334–14338.
- 52A. Shigematsu, T. Yamada, H. Kitagawa, J. Am. Chem. Soc. 2011, 133, 2034–2036.
- 53N. Agmon, Chem. Phys. Lett. 1995, 244, 456–462.
- 54K.-D. Kreuer, A. Rabenau, W. Weppner, Angew. Chem. Int. Ed. Engl. 1982, 21, 208–209.
- 55H. Endo, H. Nishide, A. Sonai, T. Tago, US 7,829,218 B2, 2010.
- 56M. W. Wong, K. B. Wiberg, M. J. Frisch, J. Am. Chem. Soc. 1992, 114, 523–529.
- 57M. Meot-Ner (Mautner), Chem. Rev. 2005, 105, 213–284.
- 58S. Liao, D. Qiu, P. Yi, L. Peng, X. Lai, Energy 2022, 261, 125235.
- 59B. Xiao, J. Zhao, L. Fan, Y. Liu, S. H. Chan, Z. Tu, Energy 2022, 261, 123298.
- 60G. Férey, C. Mellot-Draznieks, C. Serre, F. Millange, J. Dutour, S. Surble, I. Margiolaki, Science 2005, 309, 2040–2042.