An Electron-Deficient CpE Iridium(III) Catalyst: Synthesis, Characterization, and Application to Ether-Directed C−H Amidation
Eiki Tomita
Faculty of Pharmaceutical Sciences, Hokkaido University, Kita-ku, Sapporo, 060-0812 Japan
Search for more papers by this authorDr. Masahiro Kojima
Faculty of Pharmaceutical Sciences, Hokkaido University, Kita-ku, Sapporo, 060-0812 Japan
Search for more papers by this authorDr. Yuki Nagashima
Department of Chemical Science and Engineering, Tokyo Institute of Technology, O-okayama, Meguro-ku, Tokyo, 152-8550 Japan
Search for more papers by this authorProf. Dr. Ken Tanaka
Department of Chemical Science and Engineering, Tokyo Institute of Technology, O-okayama, Meguro-ku, Tokyo, 152-8550 Japan
Search for more papers by this authorDr. Haruki Sugiyama
Institute for Molecular Science, Okazaki, 444-8787 Japan
Department of Structural Molecular Science, SOKENDAI (The Graduate University for Advanced Studies), Myodaiji, Okazaki, 444-8787 Japan
Search for more papers by this authorProf. Dr. Yasutomo Segawa
Institute for Molecular Science, Okazaki, 444-8787 Japan
Department of Structural Molecular Science, SOKENDAI (The Graduate University for Advanced Studies), Myodaiji, Okazaki, 444-8787 Japan
Search for more papers by this authorDr. Atsushi Furukawa
Faculty of Pharmaceutical Sciences, Hokkaido University, Kita-ku, Sapporo, 060-0812 Japan
Current address: Faculty of Pharmaceutical Sciences, Institute of Medical, Pharmaceutical and Health Sciences, Kanazawa University, Kanazawa, 920-1192 Japan
Search for more papers by this authorProf. Dr. Katsumi Maenaka
Faculty of Pharmaceutical Sciences, Hokkaido University, Kita-ku, Sapporo, 060-0812 Japan
Global Station for Biosurfaces and Drug Discovery, Hokkaido University, Kita-ku, Sapporo, 060-0812 Japan
Search for more papers by this authorProf. Dr. Satoshi Maeda
Institute for Chemical Reaction Design and Discovery (WPI-ICReDD), Hokkaido University, Kita-ku, Sapporo 001-0021, Japan
Department of Chemistry, Faculty of Science, Hokkaido University, Kita-ku, Sapporo, 060-0810 Japan
JST, ERATO Maeda Artificial Intelligence for Chemical Reaction Design and Discovery Project, Kita-ku, Sapporo, 060-0810 Japan
Search for more papers by this authorCorresponding Author
Dr. Tatsuhiko Yoshino
Faculty of Pharmaceutical Sciences, Hokkaido University, Kita-ku, Sapporo, 060-0812 Japan
Global Station for Biosurfaces and Drug Discovery, Hokkaido University, Kita-ku, Sapporo, 060-0812 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Shigeki Matsunaga
Faculty of Pharmaceutical Sciences, Hokkaido University, Kita-ku, Sapporo, 060-0812 Japan
Global Station for Biosurfaces and Drug Discovery, Hokkaido University, Kita-ku, Sapporo, 060-0812 Japan
Search for more papers by this authorEiki Tomita
Faculty of Pharmaceutical Sciences, Hokkaido University, Kita-ku, Sapporo, 060-0812 Japan
Search for more papers by this authorDr. Masahiro Kojima
Faculty of Pharmaceutical Sciences, Hokkaido University, Kita-ku, Sapporo, 060-0812 Japan
Search for more papers by this authorDr. Yuki Nagashima
Department of Chemical Science and Engineering, Tokyo Institute of Technology, O-okayama, Meguro-ku, Tokyo, 152-8550 Japan
Search for more papers by this authorProf. Dr. Ken Tanaka
Department of Chemical Science and Engineering, Tokyo Institute of Technology, O-okayama, Meguro-ku, Tokyo, 152-8550 Japan
Search for more papers by this authorDr. Haruki Sugiyama
Institute for Molecular Science, Okazaki, 444-8787 Japan
Department of Structural Molecular Science, SOKENDAI (The Graduate University for Advanced Studies), Myodaiji, Okazaki, 444-8787 Japan
Search for more papers by this authorProf. Dr. Yasutomo Segawa
Institute for Molecular Science, Okazaki, 444-8787 Japan
Department of Structural Molecular Science, SOKENDAI (The Graduate University for Advanced Studies), Myodaiji, Okazaki, 444-8787 Japan
Search for more papers by this authorDr. Atsushi Furukawa
Faculty of Pharmaceutical Sciences, Hokkaido University, Kita-ku, Sapporo, 060-0812 Japan
Current address: Faculty of Pharmaceutical Sciences, Institute of Medical, Pharmaceutical and Health Sciences, Kanazawa University, Kanazawa, 920-1192 Japan
Search for more papers by this authorProf. Dr. Katsumi Maenaka
Faculty of Pharmaceutical Sciences, Hokkaido University, Kita-ku, Sapporo, 060-0812 Japan
Global Station for Biosurfaces and Drug Discovery, Hokkaido University, Kita-ku, Sapporo, 060-0812 Japan
Search for more papers by this authorProf. Dr. Satoshi Maeda
Institute for Chemical Reaction Design and Discovery (WPI-ICReDD), Hokkaido University, Kita-ku, Sapporo 001-0021, Japan
Department of Chemistry, Faculty of Science, Hokkaido University, Kita-ku, Sapporo, 060-0810 Japan
JST, ERATO Maeda Artificial Intelligence for Chemical Reaction Design and Discovery Project, Kita-ku, Sapporo, 060-0810 Japan
Search for more papers by this authorCorresponding Author
Dr. Tatsuhiko Yoshino
Faculty of Pharmaceutical Sciences, Hokkaido University, Kita-ku, Sapporo, 060-0812 Japan
Global Station for Biosurfaces and Drug Discovery, Hokkaido University, Kita-ku, Sapporo, 060-0812 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Shigeki Matsunaga
Faculty of Pharmaceutical Sciences, Hokkaido University, Kita-ku, Sapporo, 060-0812 Japan
Global Station for Biosurfaces and Drug Discovery, Hokkaido University, Kita-ku, Sapporo, 060-0812 Japan
Search for more papers by this authorGraphical Abstract
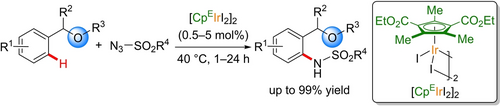
A highly electron-deficient cyclopentadienyl iridium(III) complex was developed ([CpEIrI2]2). This complex effectively catalyzed weakly coordinating ether-directed C−H amidation reactions under mild reaction conditions. Mechanistic experiments and DFT calculations indicated that the high catalytic activity of the [CpEIrI2]2 complex is attributed to its highly electron-deficient nature.
Abstract
The synthesis, characterization, and catalytic performance of an iridium(III) catalyst with an electron-deficient cyclopentadienyl ligand ([CpEIrI2]2) are reported. The [CpEIrI2]2 catalyst was synthesized by complexation of a precursor of the CpE ligand with [Ir(cod)OAc]2, followed by oxidation, desilylation, and removal of the COD ligand. The electron-deficient [CpEIrI2]2 catalyst enabled C−H amidation reactions assisted by a weakly coordinating ether directing group. Experimental mechanistic studies and DFT calculations suggested that the high catalytic performance of [CpEIrI2]2 is due to its electron-deficient nature, which accelerates both C−H activation and IrV-nitrenoid formation.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202301259-sup-0001-cartesian_coordinates.xyz453.3 KB | Supporting Information |
| anie202301259-sup-0001-HSU097_3.cif2.4 MB | Supporting Information |
| anie202301259-sup-0001-misc_information.pdf13.5 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aB. M. Trost, Science 1991, 254, 1471–1477;
- 1bP. A. Wender, B. L. Miller, Nature 2009, 460, 197–201.
- 2
- 2aK. Ueura, T. Satoh, M. Miura, Org. Lett. 2007, 9, 1407–1409;
- 2bT. Satoh, M. Miura, Chem. Eur. J. 2010, 16, 11212–11222;
- 2cN. Kuhl, N. Schröder, F. Glorius, Adv. Synth. Catal. 2014, 356, 1443–1460;
- 2dG. Song, X. Li, Acc. Chem. Res. 2015, 48, 1007–1020;
- 2eK. Shin, H. Kim, S. Chang, Acc. Chem. Res. 2015, 48, 1040–1052;
- 2fT. Yoshino, S. Matsunaga, Adv. Synth. Catal. 2017, 359, 1245–1262;
- 2gX. Li, W. Ouyang, J. Nie, S. Ji, Q. Chen, Y. Huo, ChemCatChem 2020, 12, 2358–2384.
- 3J. F. Hartwig, Organotransition Metal Chemistry: From Bonding to Catalysis, University Science Books, Sausalito, 2010.
- 4For selected examples of regioselective C−H functionalization reactions catalyzed by modified CpRhIII catalysts, see:
- 4aT. K. Hyster, T. Rovis, Chem. Sci. 2011, 2, 1606–1610;
- 4bT. K. Hyster, T. Rovis, Chem. Commun. 2011, 47, 11846–11848;
- 4cM. D. Wodrich, B. Ye, J. F. Gonthier, C. Corminboeuf, N. Cramer, Chem. Eur. J. 2014, 20, 15409–15418;
- 4dT. K. Hyster, D. M. Dalton, T. Rovis, Chem. Sci. 2015, 6, 254–258;
- 4eB. Li, J. Yang, H. Xu, H. Song, B. Wang, J. Org. Chem. 2015, 80, 12397–12409;
- 4fE. A. Trifonova, N. M. Ankudinov, M. V. Kozlov, M. Y. Sharipov, Y. V. Nelyubina, D. S. Perekalin, Chem. Eur. J. 2018, 24, 16570–16575;
- 4gJ. Terasawa, Y. Shibata, Y. Kimura, K. Tanka, Chem. Asian J. 2018, 13, 505–509;
- 4hJ. S. Barber, S. Scales, M. Tran-Dubé, F. Wang, N. W. Sach, L. Bernier, M. R. Collins, J. Zhu, I. J. McAlpine, R. L. Patman, Org. Lett. 2019, 21, 5689–5693;
- 4iS. Lee, N. Semakul, T. Rovis, Angew. Chem. Int. Ed. 2020, 59, 4965–4969.
- 5For selected examples of diastereoselective C−H functionalization reactions catalyzed by modified CpRhIII catalysts, see:
- 5aT. Piou, T. Rovis, J. Am. Chem. Soc. 2014, 136, 11292–11295;
- 5bN. Semakul, K. E. Jackson, R. S. Paton, T. Rovis, Chem. Sci. 2017, 8, 1015–1020;
- 5cT. Piou, F. Romanov-Michailidis, M. A. Ashley, M. Romanova-Michaelides, T. Rovis, J. Am. Chem. Soc. 2018, 140, 9587–9593;
- 5dF. Burg, T. Rovis, J. Am. Chem. Soc. 2021, 143, 17964–17969;
- 5eF. Burg, T. Rovis, ACS Catal. 2022, 12, 9690–9697.
- 6H. Lei, T. Rovis, Nat. Chem. 2020, 12, 725–731.
- 7
- 7aT. Piou, T. Rovis, Nature 2015, 527, 86–90;
- 7bL. Li, H. Gao, M. Sun, Z. Zhou, W. Yi, Org. Lett. 2020, 22, 5473–5478.
- 8For selected examples of enantioselective C−H functionalization reactions catalyzed by chiral CpMIII catalysts, see:
- 8aT. K. Hyster, L. Knörr, T. R. Ward, T. Rovis, Science 2012, 338, 500–503;
- 8bB. Ye, N. Cramer, Science 2012, 338, 504–506;
- 8cB. Ye, N. Cramer, J. Am. Chem. Soc. 2013, 135, 636–639;
- 8dM. Dieckmann, Y.-S. Jang, N. Cramer, Angew. Chem. Int. Ed. 2015, 54, 12149–12152;
- 8eJ. Zheng, W.-J. Cui, C. Zheng, S.-L. You, J. Am. Chem. Soc. 2016, 138, 5242–5245;
- 8fZ.-J. Jia, C. Merten, R. Gontla, C. G. Daniliuc, A. P. Antonchick, H. Waldmann, Angew. Chem. Int. Ed. 2017, 56, 2429–2434;
- 8gE. A. Trifonova, N. M. Ankudinov, A. A. Mikhaylov, D. A. Chusov, Y. V. Nelyubina, D. S. Perekalin, Angew. Chem. Int. Ed. 2018, 57, 7714–7718;
- 8hK. Ozols, Y.-S. Jang, N. Cramer, J. Am. Chem. Soc. 2019, 141, 5675–5680;
- 8iG. Li, X. Yan, J. Jiang, H. Liang, C. Zhou, J. Wang, Angew. Chem. Int. Ed. 2020, 59, 22436–22440;
- 8jC. M. B. Farr, A. M. Kazerouni, B. Park, C. D. Poff, J. Won, K. R. Sharp, M.-H. Baik, S. B. Blakey, J. Am. Chem. Soc. 2020, 142, 13996–14004;
- 8kJ. Mas-Roselló, A. G. Herraiz, B. Audic, A. Laverny, N. Cramer, Angew. Chem. Int. Ed. 2021, 60, 13198–13224;
- 8lX. Yan, J. Jiang, J. Wang, Angew. Chem. Int. Ed. 2022, 61, e202201522;
- 8mX. Yan, J. Wang, Synthesis 2023, https://doi.org/10.1055/a-2005-5006.
- 9For selected examples of enantioselective C−H functionalization reactions catalyzed by achiral modified CpMIII catalysts with chiral carboxylic acids, see:
- 9aL. Lin, S. Fukagawa, D. Sekine, E. Tomita, T. Yoshino, S. Matsunaga, Angew. Chem. Int. Ed. 2018, 57, 12048–12052;
- 9bS. Fukagawa, Y. Kato, R. Tanaka, M. Kojima, T. Yoshino, S. Matsunaga, Angew. Chem. Int. Ed. 2019, 58, 1153–1157;
- 9cW. Liu, W. Yang, J. Zhu, Y. Guo, N. Wang, J. Ke, P. Yu, C. He, ACS Catal. 2020, 10, 7207–7215;
- 9dT. Yoshino, S. Matsunaga, ACS Catal. 2021, 11, 6455–6466.
- 10
- 10aT. A. Davis, C. Wang, T. Rovis, Synlett 2015, 26, 1520–1524;
- 10bW. Lin, W. Li, D. Lu, F. Su, T.-B. Wen, H.-J. Zhang, ACS Catal. 2018, 8, 8070–8076;
- 10cR. Yoshimura, K. Tanaka, Chem. Eur. J. 2020, 26, 4969–4973;
- 10dJ. Tanaka, Y. Nagashima, K. Tanaka, Org. Lett. 2020, 22, 7181–7186;
- 10eV. B. Kharitonov, D. V. Muratov, Y. V. Nelyubina, D. A. Loginov, Synthesis 2022, 54, 5119–5127;
- 10fN. Wagner-Carlberg, T. Rovis, J. Am. Chem. Soc. 2022, 144, 22426–22432;
- 10gM. Peng, C.-S. Wang, P.-P. Chen, T. Roisnel, H. Doucet, K. N. Houk, J.-F. Soulé, J. Am. Chem. Soc. 2023, 145, 4508–4516.
- 11
- 11aS. Y. Hong, J. Jeong, S. Chang, Angew. Chem. Int. Ed. 2017, 56, 2408–2412;
- 11bT. Yamada, Y. Shibata, S. Kawauchi, S. Yoshizaki, K. Tanaka, Chem. Eur. J. 2018, 24, 5723–5727;
- 11cY. Honjo, Y. Shibata, K. Tanaka, Chem. Eur. J. 2019, 25, 9427–9432;
- 11dT. Yamada, Y. Shibata, K. Tanaka, Chem. Eur. J. 2019, 25, 16022–16031.
- 12For a review on the modified CpRhIII complexes, see: T. Piou, T. Rovis, Acc. Chem. Res. 2018, 51, 170–180.
- 13
- 13aJ. M. Neely, T. Rovis, J. Am. Chem. Soc. 2014, 136, 2735–2738;
- 13bF. Romanov-Michailidis, K. F. Sedillo, J. M. Neely, T. Rovis, J. Am. Chem. Soc. 2015, 137, 8892–8895;
- 13cE. J. T. Phipps, T. Rovis, J. Am. Chem. Soc. 2019, 141, 6807–6811;
- 13dE. J. T. Phipps, T. Piou, T. Rovis, Synlett 2019, 30, 1787–1790.
- 14
- 14aS. Yoshizaki, Y. Shibata, K. Tanaka, Angew. Chem. Int. Ed. 2017, 56, 3590–3593;
- 14bT. Yamada, Y. Shibata, K. Tanaka, Asian J. Org. Chem. 2018, 7, 1396–1402;
- 14cR. Yoshimura, Y. Shibata, T. Yamada, K. Tanaka, J. Org. Chem. 2019, 84, 2501–2511;
- 14dR. Yoshimura, Y. Shibata, S. Yoshizaki, J. Terasawa, T. Yamada, K. Tanaka, Asian J. Org. Chem. 2019, 8, 986–993;
- 14eJ. Tanaka, Y. Shibata, A. Joseph, J. Nogami, J. Terasawa, R. Yoshimura, K. Tanaka, Chem. Eur. J. 2020, 26, 5774–5779;
- 14fJ. Tanaka, Y. Nagashima, A. J. Araujo Dias, K. Tanaka, J. Am. Chem. Soc. 2021, 143, 11325–11331.
- 15
- 15aY. Shibata, K. Tanaka, Angew. Chem. Int. Ed. 2011, 50, 10917–10921;
- 15bK. Morimoto, M. Itoh, K. Hirano, T. Satoh, Y. Shibata, K. Tanaka, M. Miura, Angew. Chem. Int. Ed. 2012, 51, 5359–5362;
- 15cY. Hoshino, Y. Shibata, K. Tanaka, Adv. Synth. Catal. 2014, 356, 1577–1585;
- 15dM. Fukui, Y. Hoshino, T. Satoh, M. Miura, K. Tanaka, Adv. Synth. Catal. 2014, 356, 1638–1644;
- 15eY. Honjo, Y. Shibata, E. Kudo, T. Namba, K. Masutomi, K. Tanaka, Chem. Eur. J. 2018, 24, 317–321;
- 15fM. Font, B. Cendón, A. Seoane, J. L. Mascareñas, M. Gulías, Angew. Chem. Int. Ed. 2018, 57, 8255–8259;
- 15gR. Yoshimura, Y. Shibata, K. Tanaka, J. Org. Chem. 2019, 84, 13164–13171;
- 15hG. Mihara, K. Ghosh, Y. Nishii, M. Miura, Org. Lett. 2020, 22, 5706–5711;
- 15iH. Takahashi, Y. Honjo, Y. Shibata, Y. Nagashima, K. Tanaka, Synthesis 2021, 53, 3065–3074;
- 15jY. Nagashima, S. Ishigaki, J. Tanaka, K. Tanaka, ACS Catal. 2021, 11, 13591–13602;
- 15kH. Gao, L. Hu, Y. Hu, X. Lv, Y.-B. Wu, G. Lu, Org. Chem. Front. 2022, 9, 979–988.
- 16
- 16aK. Ueura, T. Satoh, M. Miura, J. Org. Chem. 2007, 72, 5362–5367;
- 16bJ. Park, S. Chang, Chem. Asian J. 2018, 13, 1089–1102;
- 16cS. Yamane, T. Hinoue, Y. Usuki, M. Itazaki, H. Nakazawa, Y. Hayashi, S. Kawauchi, M. Miura, T. Satoh, Chem. Eur. J. 2018, 24, 7852–7855;
- 16dQ.-L. Yang, Y.-K. Xing, X.-Y. Wang, H.-X. Ma, X.-J. Weng, X. Yang, H.-M. Guo, T.-S. Mei, J. Am. Chem. Soc. 2019, 141, 18970–18976.
- 17F. Romanov-Michailidis, B. D. Ravetz, D. W. Paley, T. Rovis, J. Am. Chem. Soc. 2018, 140, 5370–5374.
- 18For other examples of reactions catalyzed by moderately electron-deficient CpIrIII catalysts, see:
- 18aJ. H. Conway, Jr., T. Rovis, J. Am. Chem. Soc. 2018, 140, 135–138;
- 18bH. Lei, J. H. Conway, Jr., C. C. Cook, T. Rovis, J. Am. Chem. Soc. 2019, 141, 11864–11869.
- 19E. Tomita, K. Yamada, Y. Shibata, K. Tanaka, M. Kojima, T. Yoshino, S. Matsunaga, Angew. Chem. Int. Ed. 2020, 59, 10474–10478.
- 20For the comparison of the electron-deficiency of the modified CpRhIII complexes, see: T. Piou, F. Romanov-Michailidis, M. Romanova-Michaelides, K. E. Jackson, N. Semakul, T. D. Taggart, B. S. Newell, C. D. Rithner, R. S. Paton, T. Rovis, J. Am. Chem. Soc. 2017, 139, 1296–1310.
- 21
- 21aD. J. Gorin, F. D. Toste, Nature 2007, 446, 395–403;
- 21bA. Leyva-Pérez, A. Corma, Angew. Chem. Int. Ed. 2012, 51, 614–635;
- 21cT. M. Figg, S. Park, J. Park, S. Chang, D. G. Musaev, Organometallics 2014, 33, 4076–4085;
- 21dY. Park, J. Heo, M.-H. Baik, S. Chang, J. Am. Chem. Soc. 2016, 138, 14020–14029.
- 22K. M. Engle, T.-S. Mei, M. Wasa, J.-Q. Yu, Acc. Chem. Res. 2012, 45, 788–802.
- 23
- 23aZ. Chen, B. Wang, J. Zhang, W. Yu, Z. Liu, Y. Zhang, Org. Chem. Front. 2015, 2, 1107–1295;
- 23bC. Sambiagio, D. Schönbauer, R. Blieck, T. Dan-Huy, G. Pototschnig, P. Schaaf, T. Wiesinger, M. F. Zia, J. Wencel-Delord, T. Besset, B. U. W. Maes, M. Schnürch, Chem. Soc. Rev. 2018, 47, 6603–6743;
- 23cK. Murali, L. A. Machado, R. L. Carvalho, L. F. Pedrosa, R. Mukherjee, E. N. Da Silva Júnior, D. Maiti, Chem. Eur. J. 2021, 27, 12453–12508;
- 23dR. Mandal, B. Garai, B. Sundararaju, ACS Catal. 2022, 12, 3452–3506.
- 24
- 24aA. Tomberg, M. É. Muratore, M. J. Johansson, I. Terstiege, C. Sköld, P.-O. Norrby, iScience 2019, 20, 373–391;
- 24bG. Liao, T. Zhang, L. Jin, B.-J. Wang, C.-K. Xu, Y. Lan, Y. Zhao, B.-F. Shi, Angew. Chem. Int. Ed. 2022, 61, e202115221.
- 25Á. Iglesias, R. Álvarez, Á. R. de Lera, K. Muñiz, Angew. Chem. Int. Ed. 2012, 51, 2225–2228.
- 26G. Li, D. Leow, L. Wan, J.-Q. Yu, Angew. Chem. Int. Ed. 2013, 52, 1245–1247.
- 27E. Tan, O. Quinonero, M. E. de Orbe, A. M. Echavarren, ACS Catal. 2018, 8, 2166–2172.
- 28For other examples of ether-directed C−H functionalization reactions, see:
- 28aS. Kawamorita, H. Ohmiya, K. Hara, A. Fukuoka, M. Sawamura, J. Am. Chem. Soc. 2009, 131, 5058–5059;
- 28bJ. Oyamada, M. Nishiura, Z. Hou, Angew. Chem. Int. Ed. 2011, 50, 10720–10723;
- 28cJ. Oyamada, Z. Hou, Angew. Chem. Int. Ed. 2012, 51, 12828–12832;
- 28dC. W. Liskey, J. F. Hartwig, J. Am. Chem. Soc. 2012, 134, 12422–12425;
- 28eL. Zhao, X. Shi, J. Cheng, ACS Catal. 2021, 11, 2041–2046;
- 28fM. E. Hoque, M. M. M. Hassan, B. Chattopadhyay, J. Am. Chem. Soc. 2021, 143, 5022–5037;
- 28gL. Zhao, P. Deng, X. Gong, X. Kang, J. Cheng, ACS Catal. 2022, 12, 7877–7885;
- 28hS. Wang, C. Zhu, L. Ning, D. Li, X. Feng, S. Dong, Chem. Sci. 2023, 14, 3132–3139;
- 28iT. Xie, L. Chen, Z. Shen, S. Xu, Angew. Chem. Int. Ed. 2023, 62, e202300199.
- 29B. Audic, M. D. Wodrich, N. Cramer, Chem. Sci. 2019, 10, 781–787.
- 30D. A. Loginov, A. M. Miloserdov, Z. A. Starikova, P. V. Petrovskii, A. R. Kudinov, Mendeleev Commun. 2012, 22, 192–193.
- 31
- 31aR. P. Hughes, D. C. Lindner, L. M. Liable-Sands, A. L. Rheingold, Organometallics 2001, 20, 363–366;
- 31bJ. Yuan, R. P. Hughes, A. L. Rheingold, Inorg. Chim. Acta 2010, 364, 96–101.
- 32Deposition Number 2123310 (for 9) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 33For selected examples of C−H amidation reactions using sulfonyl azides as a reactant, see:
- 33aJ. Y. Kim, S. H. Park, J. Ryu, S. H. Cho, S. H. Kim, S. Chang, J. Am. Chem. Soc. 2012, 134, 9110–9113;
- 33bJ. Kim, J. Kim, S. Chang, Chem. Eur. J. 2013, 19, 7328–7333;
- 33cM. R. Yadav, R. K. Rit, A. K. Sahoo, Org. Lett. 2013, 15, 1638–1641;
- 33dQ.-Z. Zheng, Y.-F. Liang, C. Qin, N. Jiao, Chem. Commun. 2013, 49, 5654–5656;
- 33eV. S. Thirunavukkarasu, K. Raghuvanshi, L. Ackermann, Org. Lett. 2013, 15, 3286–3289;
- 33fD. Lee, Y. Kim, S. Chang, J. Org. Chem. 2013, 78, 11102–11109;
- 33gJ. Kim, S. Chang, Angew. Chem. Int. Ed. 2014, 53, 2203–2207;
- 33hT. Kang, Y. Kim, D. Lee, Z. Wang, S. Chang, J. Am. Chem. Soc. 2014, 136, 4141–4144.
- 34B. Sun, T. Yoshino, S. Matsunaga, M. Kanai, Adv. Synth. Catal. 2014, 356, 1491–1495.
- 35
- 35aU. Mayer, V. Gutmann, W. Gerger, Monatsh. Chem. 1975, 106, 1235–1257;
- 35bM. A. Beckett, G. C. Strickland, J. R. Holland, K. S. Varma, Polymer 1996, 37, 4629–4631;
- 35cG. C. Welch, L. Cabrera, P. A. Chase, E. Hollink, J. D. Masuda, P. Wei, D. W. Stephan, Dalton Trans. 2007, 3407–3414.
- 36E. M. Simmons, J. F. Hartwig, Angew. Chem. Int. Ed. 2012, 51, 3066–3072.
- 37To shed light on the IrV-nitrenoid formation process, Hammett plot analysis was conducted for a series of para-substituted sulfonyl azides (Figure S13). As the result, a relatively small negative ρ (−0.11) was observed, which implied that electron-donating groups on sulfonyl azides accelerated this reaction, but the influence was moderate compared to the related C−H amidation reaction in which a primary KIE was not observed. See: Y.-F. Zhang, B. Wu, Z.-J. Shi, Chem. Eur. J. 2016, 22, 17808–17812.
- 38
- 38aD. Lapointe, K. Fagnou, Chem. Lett. 2010, 39, 1118–1126;
- 38bL. Ackermann, Chem. Rev. 2011, 111, 1315–1345;
- 38cD. L. Davies, S. A. Macgregor, C. L. McMullin, Chem. Rev. 2017, 117, 8649–8709.
- 39Our preliminary calculation suggested that the Ir-X might be better described as an IrIII-nitrene or an IrIV-nitrene radical rather than IrV-nitrenoid due to the ligand field inversion, and the CpEIr complex tends to adopt a lower oxidation state than the Cp*Ir complex (for details, see the Supporting Information). For the ligand field inversion, see:
- 39aR. Hoffmann, S. Alvarez, C. Mealli, A. Falceto, T. J. Cahill III, T. Zeng, G. Manca, Chem. Rev. 2016, 116, 8173–8192;
- 39bK. M. Carsch, I. M. DiMucci, D. A. Iovan, A. Li, S.-L. Zheng, C. J. Titus, S. J. Lee, K. D. Irwin, D. Nordlund, K. M. Lancaster, T. A. Betley, Science 2019, 365, 1138–1143.
- 40Additional calculation results were shown in the Supporting Information.
- 41J.-B. Liu, X.-H. Sheng, C.-Z. Sun, F. Huang, D.-Z. Chen, ACS Catal. 2016, 6, 2452–2461.