Practical Access to meta-Substituted Anilines by Amination of Quinone Imine Ketals Derived from Anisidines: Efficient Synthesis of Anti-Psychotic Drugs
Graphical Abstract
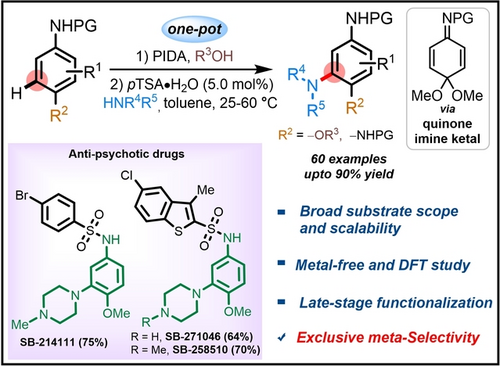
The Brønsted acid-catalyzed meta-amination of arylamines with aliphatic, heterocyclic and aromatic amines is reported based on a one-pot procedure. The strategy is based on direct C−N bond formation for the practical synthesis of meta-substituted anilines, thus reversing the conventional site-selectivity. A concise and efficient synthesis of anti-psychotics and in particular of anti-schizophrenic drugs is shown.
Abstract
Reversing the conventional site-selectivity of C−H activation provides efficient retrosynthetic disconnections to otherwise unreactive bonds. Described herein is the Brønsted acid-catalyzed reaction that selectively performs meta-amination of anisidines with aliphatic-, heterocyclic- and aromatic amines in a one-pot procedure. In addition to dramatically simplifying the synthesis of meta-substituted anilines, our approach has the advantage of the scalability and remarkable functional group tolerance, including late-stage functionalization of pharmaceutical compounds and natural products. The control experiments and detailed computational analysis provide insight into the reaction mechanism and the origin of meta-selectivity. The protocol extended to the synthesis of challenging tri-aminated arenes and successfully applied for the efficient synthesis of 5-HT6 receptor antagonists, the anti-psychotic drugs viz.. SB-214111, SB-258510, and specifically, anti-schizophrenic drug SB-271046.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.