Direct Catalytic Asymmetric and Regiodivergent N1- and C3-Allenylic Alkylation of Indoles
Taochun Zha
Key Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education, School of Chemical Science and Technology, Yunnan Provincial Center for Research & Development of Natural Products, State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Yunnan University, Kunming, 650091 China
Search for more papers by this authorJiehui Rui
Key Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education, School of Chemical Science and Technology, Yunnan Provincial Center for Research & Development of Natural Products, State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Yunnan University, Kunming, 650091 China
Search for more papers by this authorZhihan Zhang
Department of Chemistry and Shenzhen Grubbs Institute, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorDongqiang Zhang
Key Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education, School of Chemical Science and Technology, Yunnan Provincial Center for Research & Development of Natural Products, State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Yunnan University, Kunming, 650091 China
Search for more papers by this authorZhirong Yang
Key Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education, School of Chemical Science and Technology, Yunnan Provincial Center for Research & Development of Natural Products, State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Yunnan University, Kunming, 650091 China
Search for more papers by this authorCorresponding Author
Peiyuan Yu
Department of Chemistry and Shenzhen Grubbs Institute, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorYingcheng Wang
Key Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education, School of Chemical Science and Technology, Yunnan Provincial Center for Research & Development of Natural Products, State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Yunnan University, Kunming, 650091 China
Search for more papers by this authorFangzhi Peng
Key Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education, School of Chemical Science and Technology, Yunnan Provincial Center for Research & Development of Natural Products, State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Yunnan University, Kunming, 650091 China
Search for more papers by this authorCorresponding Author
Prof. Zhihui Shao
Key Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education, School of Chemical Science and Technology, Yunnan Provincial Center for Research & Development of Natural Products, State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Yunnan University, Kunming, 650091 China
Search for more papers by this authorTaochun Zha
Key Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education, School of Chemical Science and Technology, Yunnan Provincial Center for Research & Development of Natural Products, State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Yunnan University, Kunming, 650091 China
Search for more papers by this authorJiehui Rui
Key Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education, School of Chemical Science and Technology, Yunnan Provincial Center for Research & Development of Natural Products, State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Yunnan University, Kunming, 650091 China
Search for more papers by this authorZhihan Zhang
Department of Chemistry and Shenzhen Grubbs Institute, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorDongqiang Zhang
Key Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education, School of Chemical Science and Technology, Yunnan Provincial Center for Research & Development of Natural Products, State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Yunnan University, Kunming, 650091 China
Search for more papers by this authorZhirong Yang
Key Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education, School of Chemical Science and Technology, Yunnan Provincial Center for Research & Development of Natural Products, State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Yunnan University, Kunming, 650091 China
Search for more papers by this authorCorresponding Author
Peiyuan Yu
Department of Chemistry and Shenzhen Grubbs Institute, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorYingcheng Wang
Key Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education, School of Chemical Science and Technology, Yunnan Provincial Center for Research & Development of Natural Products, State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Yunnan University, Kunming, 650091 China
Search for more papers by this authorFangzhi Peng
Key Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education, School of Chemical Science and Technology, Yunnan Provincial Center for Research & Development of Natural Products, State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Yunnan University, Kunming, 650091 China
Search for more papers by this authorCorresponding Author
Prof. Zhihui Shao
Key Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education, School of Chemical Science and Technology, Yunnan Provincial Center for Research & Development of Natural Products, State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Yunnan University, Kunming, 650091 China
Search for more papers by this authorGraphical Abstract
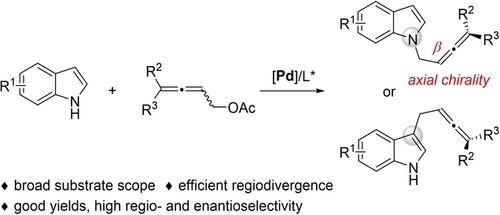
We report the first direct catalytic asymmetric N1-functionalization of 1H-indoles via an allenylic alkylation strategy. This transformation produces N-alkylated indoles bearing axial chirality with a stereocenter non-adjacent (β) to the nitrogen. The regioselectivity (N1/C3) of this process can be switched efficiently. We also introduce a new class of tri-substituted allenylic electrophiles.
Abstract
Herein we report a Pd-catalyzed asymmetric allenylic alkylation strategy for the direct functionalization of 1H-indoles by employing P-chiral BIBOP-type ligands. The regioselectivity (N1/C3) of this process can be switched efficiently. Using Cs2CO3 at elevated temperatures in MeCN, N1-alkylated indoles bearing axial chirality with a stereocenter non-adjacent (β) to the nitrogen are produced in good yields with high enantioselectivity and complete N1-regioselectivity regardless of the electronic properties and substitution patterns of diverse indoles. Using K2CO3 at room temperature in CH2Cl2, chiral C3-alkylated indoles can also be obtained. Notably, we introduce a new class of tri-substituted allenylic electrophiles that proceeded through different pathways from di-substituted allenylic electrophiles.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202300844-sup-0001-11.cif1,017.4 KB | Supporting Information |
| anie202300844-sup-0001-3ag.cif600.6 KB | Supporting Information |
| anie202300844-sup-0001-4j.cif769.8 KB | Supporting Information |
| anie202300844-sup-0001-7a.cif501.6 KB | Supporting Information |
| anie202300844-sup-0001-misc_information.pdf28.9 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aW. Gul, M. T. Hamann, Life Sci. 2005, 78, 442–453;
- 1bS. E. O'Connor, J. J. Maresh, Nat. Prod. Rep. 2006, 23, 532–547;
- 1cT. Sravanthi, S. Manju, Eur. J. Pharm. Sci. 2016, 91, 1–10;
- 1dE. Vitaku, D. T. Smith, J. T. Njardarson, J. Med. Chem. 2014, 57, 10257–10274.
- 2For selected reviews, see:
- 2aM. Bandini, A. Eichholzer, Angew. Chem. Int. Ed. 2009, 48, 9608–9644;
- 2bS.-L. You, Q. Cai, M. Zeng, Chem. Soc. Rev. 2009, 38, 2190–2201;
- 2cG. Bartoli, G. Bencivenni, R. Dalpozzo, Chem. Soc. Rev. 2010, 39, 4449–4465;
- 2dJ.-B. Chen, Y.-X. Jia, Org. Biomol. Chem. 2017, 15, 3550–3567.
- 3
- 3aS. Lakhdar, M. Westermaier, F. Terrier, R. Goumont, T. Boubaker, A. R. Ofial, H. Mayr, J. Org. Chem. 2006, 71, 9088–9095;
- 3bN. Otero, M. Mandado, R. A. Mosquera, J. Phys. Chem. A 2007, 111, 5557–5562.
- 4For a recent review, see: D. Trubitsõn, T. Kanger, Symmetry 2020, 12, 1184.
- 5For selected examples of metal-catalyzed asymmetric N1-alkylation of indoles, see:
- 5aL. M. Stanley, J. F. Hartwig, Angew. Chem. Int. Ed. 2009, 48, 7841–7844;
- 5bB. M. Trost, M. Osipov, G. Dong, J. Am. Chem. Soc. 2010, 132, 15800–15807;
- 5cH.-G. Cheng, L.-Q. Lu, T. Wang, Q.-Q. Yang, X.-P. Liu, Y. Li, Q.-H. Deng, J.-R. Chen, W.-J. Xiao, Angew. Chem. Int. Ed. 2013, 52, 3250–3254;
- 5dL.-Y. Chen, X.-Y. Yu, J.-R. Chen, B. Feng, H. Zhang, Y.-H. Qi, W.-J. Xiao, Org. Lett. 2015, 17, 1381–1384;
- 5eQ. M. Kainz, C. D. Matier, A. Bartoszewicz, S. L. Zultanski, J. C. Peters, G. C. Fu, Science 2016, 351, 681–684;
- 5fK. Y. Ye, Q. Cheng, C. X. Zhuo, L. X. Dai, S. L. You, Angew. Chem. Int. Ed. 2016, 55, 8113–8116;
- 5gB. M. Trost, E. Gnanamani, C. I. Hung, Angew. Chem. Int. Ed. 2017, 56, 10451–10456;
- 5hJ. R. Allen, A. Bahamonde, Y. Furukawa, M. S. Sigman, J. Am. Chem. Soc. 2019, 141, 8670–8674;
- 5iS. W. Kim, T. T. Schempp, J. R. Zbieg, C. E. Stivala, M. J. Krische, Angew. Chem. Int. Ed. 2019, 58, 7762–7766;
- 5jJ. Wei, B. Cao, C.-W. Tse, X.-Y. Chang, C.-Y. Zhou, C.-M. Che, Chem. Sci. 2020, 11, 684–693;
- 5kM. Sun, M. Liu, C. Li, Chem. Eur. J. 2021, 27, 3457–3462.
- 6For selected examples of organocatalyzed asymmetric N1-alkylation of indoles, see:
- 6aY. Wang, S. Wang, W. Shan, Z. Shao, Nat. Commun. 2020, 11, 226;
- 6bL. Zhang, B. Wu, Z. Chen, J. Hu, X. Zeng, G. Zhong, Chem. Commun. 2018, 54, 9230–9233;
- 6cM. Chen, J. Sun, Angew. Chem. Int. Ed. 2017, 56, 4583–4587;
- 6dH.-L. Cui, X. Feng, J. Peng, J. Lei, K. Jiang, Y.-C. Chen, Angew. Chem. Int. Ed. 2009, 48, 5737–5740;
- 6eM. Bandini, A. Eichholzer, M. Tragni, A. Umani-Ronchi, Angew. Chem. Int. Ed. 2008, 47, 3238–3241.
- 7
- 7aW. B. Liu, X. Zhang, L. X. Dai, S. L. You, Angew. Chem. Int. Ed. 2012, 51, 5183–5187;
- 7bK. Xu, T. Gilles, B. Breit, Nat. Commun. 2015, 6, 7616;
- 7cY. Ye, S.-T. Kim, J. Jeong, M.-H. Baik, S. L. Buchwald, J. Am. Chem. Soc. 2019, 141, 3901–3909;
- 7dX. Li, W. Wang, Q. He, Y. Liu, R. Fan, Green Synth. Catal. 2022, 3, 282–286;
- 7eL. Li, J. Ren, J. Zhou, X. Wu, Z. Shao, X. Yang, D. Qian, Nat. Commun. 2022, 13, 6861.
- 8
- 8aRef. [6a];
- 8bRef. [7e];
- 8cY. Wang, L. Jiang, L. Li, J. Dai, D. Xiong, Z. Shao, Angew. Chem. Int. Ed. 2016, 55, 15142–15146.
- 9For selected reviews, see:
- 9aC. Nájera, I. P. Beletskaya, M. Yus, Chem. Soc. Rev. 2019, 48, 4515–4618;
- 9bN. Funken, Y.-Q. Zhang, A. Gansäuer, Chem. Eur. J. 2017, 23, 19–32.
- 10For selected examples, see:
- 10aS. Xu, Z.-M. Zhang, B. Xu, B. Liu, Y. Liu, J. Zhang, J. Am. Chem. Soc. 2018, 140, 2272–2283;
- 10bL. Li, P. Luo, Y. Deng, Z. Shao, Angew. Chem. Int. Ed. 2019, 58, 4710–4713;
- 10cL.-C. Yang, Y.-N. Wang, R. Liu, Y. Luo, X. Q. Ng, B. Yang, Z.-Q. Rong, Y. Lan, Z. Shao, Y. Zhao, Nat. Chem. 2020, 12, 860–868;
- 10dB. Wu, J. R. Parquette, T. V. RajanBabu, Science 2009, 326, 1662;
- 10eY. Tani, T. Fujihara, J. Terao, Y. Tsuji, J. Am. Chem. Soc. 2014, 136, 17706–17709;
- 10fS. R. Sardini, M. K. Brown, J. Am. Chem. Soc. 2017, 139, 9823–9826;
- 10gR. Sakae, K. Hirano, M. Miura, J. Am. Chem. Soc. 2015, 137, 6460–6463.
- 11For selected examples of metal-catalyzed enantioselective allenylic substitution leading to axially chiral allene compounds, see:
- 11aY. Imada, K. Ueno, K. Kutsuwa, S.-I. Murahashi, Chem. Lett. 2002, 31, 140–141;
- 11bB. M. Trost, D. R. Fandrick, D. C. Dinh, J. Am. Chem. Soc. 2005, 127, 14186–14187;
- 11cS. Song, J. Zhou, C. Fu, S. Ma, Nat. Commun. 2019, 10, 507;
- 11dB. M. Trost, J. E. Schultz, T. Chang, M. R. Maduabum, J. Am. Chem. Soc. 2019, 141, 9521–9526;
- 11eS. Song, S. Ma, Chin. J. Chem. 2020, 38, 1233–1238;
- 11fJ. Zhang, X. Huo, J. Xiao, L. Zhao, S. Ma, W. Zhang, J. Am. Chem. Soc. 2021, 143, 12622–12632.
- 12For selected examples of metal-catalyzed enantioselective allenylic substitution leading to centrally chiral allene compounds, see:
- 12aD. A. Petrone, M. Isomura, I. Franzoni, S. L. Rössler, E. M. Carreira, J. Am. Chem. Soc. 2018, 140, 4697–4704;
- 12bF. Glatz, D. A. Petrone, E. M. Carreira, Angew. Chem. Int. Ed. 2020, 59, 16404–16408;
- 12cM. Isomura, D. A. Petrone, E. M. Carreira, J. Am. Chem. Soc. 2021, 143, 3323–3329;
- 12dQ. Li, C. Fu, S. Ma, Angew. Chem. Int. Ed. 2012, 51, 11783–11786;
- 12eQ. Li, C. Fu, S. Ma, Angew. Chem. Int. Ed. 2014, 53, 6511–6514.
- 13For selected books and reviews, see:
- 13aN. Krause, A. S. Hashmi, Modern allene chemistry, Wiley-VCH, Weinheim, 2004;
- 13bA. Hoffmann-Röder, N. Krause, Angew. Chem. Int. Ed. 2004, 43, 1196–1216;
- 13cP. Rivera-Fuentes, F. Diederich, Angew. Chem. Int. Ed. 2012, 51, 2818–2828;
- 13dS. Ma, Chem. Rev. 2005, 105, 2829–2872;
- 13eS. Yu, S. Ma, Angew. Chem. Int. Ed. 2012, 51, 3074–3112.
- 14For selected reviews, see:
- 14aM. Ogasawara, Tetrahedron: Asymmetry 2009, 20, 259–271;
- 14bJ. Ye, S. Ma, Org. Chem. Front. 2014, 1, 1210–1224;
- 14cW.-D. Chu, Y. Zhang, J. Wang, Catal. Sci. Technol. 2017, 7, 4570–4579;
- 14dR. K. Neff, D. E. Frantz, ACS Catal. 2014, 4, 519–528;
- 14eX. Wang, X. Chen, W. Lin, P. Li, W. Li, Adv. Synth. Catal. 2022, 364, 1212–1222.
- 15For a review, see:
- 15aG. Xu, C. H. Senanayake, W. Tang, Acc. Chem. Res. 2019, 52, 1101–1112; For selected examples, see:
- 15bJ. Zhu, L. Huang, W. Dong, N. Li, X. Yu, W. P. Deng, W. Tang, Angew. Chem. Int. Ed. 2019, 58, 16119–16123;
- 15cL. Huang, J. Zhu, G. Jiao, Z. Wang, X. Yu, W. P. Deng, W. Tang, Angew. Chem. Int. Ed. 2016, 55, 4527–4531;
- 15dG. Liu, X. Liu, Z. Cai, G. Jiao, G. Xu, W. Tang, Angew. Chem. Int. Ed. 2013, 52, 4235–4238;
- 15eX. Kang, C. Qian, H. Yang, J. Shi, J. Claverie, W. Tang, Green Synth. Catal. 2022, 3, 185–189;
- 15fD. Tian, R. Xu, J. Zhu, J. Huang, W. Dong, J. Claverie, W. Tang, Angew. Chem. Int. Ed. 2021, 60, 6305–6309;
- 15gL. Li, S. Wang, P. Luo, R. Wang, Z. Wang, X. Li, Y.-H. Deng, F. Peng, Z. Shao, Nat. Commun. 2021, 12, 5667.
- 16For selected examples, see:
- 16aH.-F. Tu, X. Zhang, C. Zheng, M. Zhu, S.-L. You, Nat. Catal. 2018, 1, 601–608;
- 16bB. M. Trost, W.-J. Bai, C. Hohn, Y. Bai, J. J. Cregg, J. Am. Chem. Soc. 2018, 140, 6710–6717.
- 17A. W. Schmidt, K. R. Reddy, H.-J. Knölker, Chem. Rev. 2012, 112, 3193–3328.
- 18Deposition Numbers 2233046 (for 3ag), 2233045 (for 4j), and 2233044 (for 7a) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 19For selected reviews, see:
- 19aJ. Steinreiber, K. Faber, H. Griengl, Chem. Eur. J. 2008, 14, 8060–8072;
- 19bV. Bhat, E. R. Welin, X. Guo, B. M. Stoltz, Chem. Rev. 2017, 117, 4528–4561.
- 20For selected reviews, see:
- 20aJ. M. Keith, J. F. Larrow, E. N. Jacobsen, Adv. Synth. Catal. 2001, 343, 5–26;
- 20bH. Pellissier, Adv. Synth. Catal. 2011, 353, 1613–1666.
- 21
- 21aH. Kagan, J. Fiaud, Top. Stereochem. 1988, 18, 249–330;
- 21bM. D. Greenhalgh, J. E. Taylor, A. D. Smith, Tetrahedron 2018, 74, 5554–5560.
- 22For the determination of the absolute configuration of chiral tri-substituted allene (R)-5 a and (S)-6 a, please see the Supporting Information.
- 23The diastereomeric α-alkynylidene π-allylpalladium intermediates generated in situ from racemic tri-substituted allenylic electrophiles might interconvert relatively slower than the corresponding α-alkynylidene π-allylpalladium intermediates generated in situ from racemic di-substituted allenylic electrophiles, probably due to steric hindrance effect. In the present reaction system, the indole N1-nucleophilicity may be relatively higher than the indole C3-nucleophilicity. However, the clear reason is not at mature at this stage.
- 24
- 24aD. G. Blackmond, J. Am. Chem. Soc. 2001, 123, 545–553;
- 24bD. W. Johnson, D. A. Singleton, J. Am. Chem. Soc. 1999, 121, 9307–9312.
- 25T. Satyanarayana, S. Abraham, H. B. Kagan, Angew. Chem. Int. Ed. 2009, 48, 456–494.