Azulene-Fused Acenes
Albert Ong
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore, 117543 Singapore
Search for more papers by this authorProf. Tao Tao
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore, 117543 Singapore
School of Chemistry and Materials Science, Nanjing University of Information Science & Technology, Nanjing, 210044 P. R. China
Search for more papers by this authorCorresponding Author
Dr. Qing Jiang
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore, 117543 Singapore
Search for more papers by this authorDr. Yi Han
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore, 117543 Singapore
Search for more papers by this authorDr. Yaping Ou
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore, 117543 Singapore
Search for more papers by this authorProf. Kuo-Wei Huang
KAUST Catalysis Center and Division of Physical Science and Engineering, King Abdullah University of Science and Technology, Thuwal, 23955-6900 Saudi Arabia
Search for more papers by this authorCorresponding Author
Prof. Chunyan Chi
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore, 117543 Singapore
Search for more papers by this authorAlbert Ong
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore, 117543 Singapore
Search for more papers by this authorProf. Tao Tao
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore, 117543 Singapore
School of Chemistry and Materials Science, Nanjing University of Information Science & Technology, Nanjing, 210044 P. R. China
Search for more papers by this authorCorresponding Author
Dr. Qing Jiang
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore, 117543 Singapore
Search for more papers by this authorDr. Yi Han
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore, 117543 Singapore
Search for more papers by this authorDr. Yaping Ou
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore, 117543 Singapore
Search for more papers by this authorProf. Kuo-Wei Huang
KAUST Catalysis Center and Division of Physical Science and Engineering, King Abdullah University of Science and Technology, Thuwal, 23955-6900 Saudi Arabia
Search for more papers by this authorCorresponding Author
Prof. Chunyan Chi
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore, 117543 Singapore
Search for more papers by this authorGraphical Abstract
Abstract
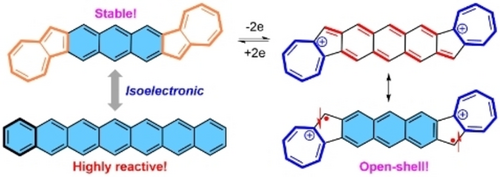
Non-alternant non-benzenoid π-conjugated polycyclic hydrocarbons (PHs) are expected to exhibit very different electronic properties from the all-benzenoid PHs. Herein, we report the synthesis and physical properties of three azulene-fused acene molecules (1, 2 and 3), which are isoelectronic to the pentacene, hexacene and heptacene, respectively. X-ray crystallographic analysis, NMR spectra, and theoretical calculations reveal a localised aromatic backbone comprising all the six- and five-membered rings while the seven-membered ring remains non-aromatic. They display properties of both azulene and acenes and are much more stable than the respective acenes. The dications of 1, 2 and 3 were formed by chemical oxidation. Notably, 32+ exhibited an open-shell diradical character (y0=30.2 %) as confirmed by variable-temperature NMR and ESR measurements, which can be explained by recovery of aromaticity of an 2,6-anthraquinodimethane unit annulated with two aromatic tropylium rings.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202209286-sup-0001-Dication_of_Molecule_3.cif1.6 MB | Supporting Information |
| anie202209286-sup-0001-misc_information.pdf6.3 MB | Supporting Information |
| anie202209286-sup-0001-Molecule_1.cif933.9 KB | Supporting Information |
| anie202209286-sup-0001-Molecule_2.cif1.2 MB | Supporting Information |
| anie202209286-sup-0001-Molecule_3.cif605.2 KB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. Bendikov, F. Wudl, D. F. Perepichka, Chem. Rev. 2004, 104, 4891;
- 1bJ. E. Anthony, Chem. Rev. 2006, 106, 5028;
- 1cJ. Wu, W. Pisula, K. Müllen, Chem. Rev. 2007, 107, 718;
- 1dJ. E. Anthony, Angew. Chem. Int. Ed. 2008, 47, 452; Angew. Chem. 2008, 120, 460;
- 1eH. F. Bettinger, C. Tönshoff, M. Doerr, E. Sanchez-Garcia, J. Chem. Theory Comput. 2016, 12, 305. f) S. Dong, A. Ong, C. Chi, J. Photochem. Photobiol. C 2019, 38, 27.
- 2
- 2aM. M. Payne, S. R. Parkin, J. E. Anthony, J. Am. Chem. Soc. 2005, 127, 8028;
- 2bD. Chun, Y. Cheng, F. Wudl, Angew. Chem. Int. Ed. 2008, 47, 8380; Angew. Chem. 2008, 120, 8508;
- 2cH. Qu, C. Chi, Org. Lett. 2010, 12, 3360;
- 2dB. Purushothaman, M. Bruzek, S. R. Parkin, A. F. Miller, J. E. Anthony, Angew. Chem. Int. Ed. 2011, 50, 7013; Angew. Chem. 2011, 123, 7151;
- 2eU. H. F. Bunz, J. U. Engelhart, B. D. Lindner, M. Schaffroth, Angew. Chem. Int. Ed. 2013, 52, 3810; Angew. Chem. 2013, 125, 3898.
- 3
- 3aQ. Ye, C. Chi, Chem. Mater. 2014, 26, 4046;
- 3bU. H. F. Bunz, Acc. Chem. Res. 2015, 48, 1676;
- 3cR. Dorel, A. M. Echavarren, Eur. J. Org. Chem. 2017, 14; d) A. N. Lakshminarayana, A. Ong, C. Chi, J. Mater. Chem. C, 2018, 6, 3551.
- 4
- 4aX. Yang, D. Liu, Q. Miao, Angew. Chem. Int. Ed. 2014, 53, 6786; Angew. Chem. 2014, 126, 6904;
- 4bX. Yang, X. Shi, N. Aratani, T. P. Gonçalves, K.-W. Huang, H. Yamada, C. Chi, Q. Miao, Chem. Sci. 2016, 7, 6176;
- 4cM. Murai, S. Iba, H. Ota, K. Takai, Org. Lett. 2017, 19, 5585;
- 4dA. Konishi, M. Yasuda, Chem. Lett. 2021, 50, 195–212.
- 5D. M. Lemal, G. D. Goldman, J. Chem. Educ. 1988, 65, 923.
- 6
- 6aM. Murai, S. Iba, H. Ota, K. Takai, Org. Lett. 2017, 19, 5585;
- 6bQ. Jiang, T. Tao, H. Phan, Y. Han, T. Y. Gopalakrishna, T. S. Herng, G. Li, L. Yuan, J. Ding, C. Chi, Angew. Chem. Int. Ed. 2018, 57, 16737; Angew. Chem. 2018, 130, 16979;
- 6cY. Sasaki, M. Takase, T. Okujima, S. Mori, H. Uno, Org. Lett. 2019, 21, 1900;
- 6dX. Yang, F. Rominger, M. Mastalerz, Angew. Chem. Int. Ed. 2019, 58, 17577; Angew. Chem. 2019, 131, 17741;
- 6eY. Han, Z. Xue, G. Li, Y. Gu, Y. Ni, S. Dong, C. Chi, Angew. Chem. Int. Ed. 2020, 59, 9026; Angew. Chem. 2020, 132, 9111;
- 6fX. Zhang, Y. Huang, J. Zhang, W. Meng, Q. Peng, R. Kong, Z. Xiao, J. Liu, M. Huang, Y. Yi, L. Chen, Q. Fan, G. Lin, Z. Liu, G. Zhang, L. Jiang, D. Zhang, Angew. Chem. Int. Ed. 2020, 59, 3529; Angew. Chem. 2020, 132, 3557;
- 6gJ. Ma, Y. Fu, E. Dmitrieva, F. Liu, H. Komber, F. Hennersdorf, A. Popov, J. Weigand, J. Liu, X. Feng, Angew. Chem. Int. Ed. 2020, 59, 5637; Angew. Chem. 2020, 132, 5686;
- 6hN. Ogawa, Y. Yamaoka, H. Takikawa, K. Yamada, K. Takasu, J. Am. Chem. Soc. 2020, 142, 13322;
- 6iB. Pigulski, K. Shoyama, F. Würthner, Angew. Chem. Int. Ed. 2020, 59, 15908; Angew. Chem. 2020, 132, 16042;
- 6jK. Horii, R. Kishi, M. Nakano, D. Shiomi, K. Sato, T. Takui, A. Konishi, M. Yasuda, J. Am. Chem. Soc. 2022, 144, 3370;
- 6kS. Wang, M. Tang, L. Wu, L. Bian, L. Jiang, J. Liu, Z. Tang, Y. Liang, Z. Liu, Angew. Chem. Int. Ed. 2022, e202205658.
- 7
- 7aY. Yamaguchi, M. Takubo, K. Ogawa, K. Nakayama, T. Koganezawa, H. Katagiri, J. Am. Chem. Soc. 2016, 138, 11335;
- 7bH. Xin, C. W. Ge, X. D. Yang, H. L. Gao, X. C. Yang, X. K. Gao, Chem. Sci. 2016, 7, 6701;
- 7cH. S. Xin, C. W. Ge, X. C. Jiao, X. D. Yang, K. Rundel, C. R. McNeill, X. K. Gao, Angew. Chem. Int. Ed. 2018, 57, 1322; Angew. Chem. 2018, 130, 1336;
- 7dH. S. Xin, J. Li, C. W. Ge, X. D. Yang, T. R. Xue, X. K. Gao, Mater. Chem. Front. 2018, 2, 975;
- 7eH. L. Gao, C. W. Ge, B. Hou, H. S. Xin, X. K. Gao, ACS Macro Lett. 2019, 8, 1360;
- 7fH. Xin, B. Hou, X. Gao, Acc. Chem. Res. 2021, 54, 1737; g) H. Xin, J. Li, R.-Q. Lu, X. Gao, T. M. Swager, J. Am. Chem. Soc., 2020, 142, 13598.
- 8S. Ito, T. Kubo, N. Morita, Y. Matsui, T. Watanabe, A. Ohta, K. Fujimori, T. Murafuji, Y. Sugiharad, A. Tajiria, Tetrahedron Lett. 2004, 45, 2891.
- 9Deposition Numbers 1993091 (for 1), 1993090 (for 2), 1993092 (for 3), and 2012401 (for 32+) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 10M. Kataoka, A. Toyota, J. Chem. Res. Synop. 1998, 278.
- 11P. v. R. Schleyer, C. Maerker, A. Dransfeld, H. Jiao, N. J. R. van Eikema Hommes, J. Am. Chem. Soc. 1996, 118, 6317.
- 12D. Geuenich, K. Hess, F. Kohler, R. Herges, Chem. Rev. 2005, 105, 3758.
- 13
- 13aS. A. Odom, S. R. Parkin, J. E. Anthony, Org. Lett. 2003, 5, 4245;
- 13bJ. E. Anthony, J. S. Brooks, D. L. Eaton, S. R. Parkin, J. Am. Chem. Soc. 2001, 123, 9482.
- 14
- 14aD. H. S. Horn, J. R. Nunn, W. S. Rapson, Nature 1947, 160, 829;
- 14bD. Sperandio, H. Hansen, Helv. Chim. Acta 1995, 78, 765.
- 15T. T. Xu, Y. Han, Z. Shen, X. Hou, Q. Jiang, W. Zeng, P. W. Ng, C. Chi, J. Am. Chem. Soc. 2021, 143, 20562.
- 16
- 16aS. Motomura, M. Nakano, H. Fukui, K. Yoneda, T. Kubo, R. Carionc, B. Champagne, Phys. Chem. Chem. Phys. 2011, 13, 20575;
- 16bR. W. A. Havenith, J. J. Engelberts, P. W. Fowler, E. Steiner, J. H. van Lenthe, P. Lazzeretti, Phys. Chem. Chem. Phys. 2004, 6, 289;
- 16cT. Nagami, J. Fujiyoshi, T. Tonami, K. Watanabe, M. Yamane, K. Okada, R. Kishi, M. Nakano, B. Champagne, V. Liégeois, Chem. Eur. J. 2018, 24, 13457.
- 17G. Rudebusch, J. Zafra, K. Jorner, K. Fukuda, J. Marshall, I. Arrechea-Marcos, G. Espejo, R. P. Ortiz, C. J. Gómez-García, L. N. Zakharov, M. Nakano, H. Ottosson, J. Casado, M. Haley, Nat. Chem. 2016, 8, 753.
- 18R. Einholz, H. F. Bettinger, Angew. Chem. Int. Ed. 2013, 52, 9818–9820; Angew. Chem. 2013, 125, 10000–10003.
- 19
- 19aZ. Zeng, X. Shi, C. Chi, J. T. L. Navarrete, J. Casado, J. Wu, Chem. Soc. Rev. 2015, 44, 6578;
- 19bS. Dong, T. S. Herng, T. Y. Gopalakrishna, H. Phan, Z. L. Lim, P. Hu, R. D. Webster, J. Ding, C. Chi, Angew. Chem. Int. Ed. 2016, 55, 9316;
- 19cT. Y. Gopalakrishna, W. Zeng, X. Lu, J. Wu, Chem. Commun. 2018, 54, 2186.
- 20B. Bleaney, K. D. Bowers, Proc. R. Soc. London Ser. A 1952, 214, 451.