Site-Selective Functionalization of Sila-Adamantane and Its Ensuing Optical Effects
Correction(s) for this article
-
Corrigendum: Site-Selective Functionalization of Sila-Adamantane and Its Ensuing Optical Effects
- Timothy C. Siu,
- M. Imex Aguirre Cardenas,
- Jacob Seo,
- Kirllos Boctor,
- Miku G. Shimono,
- Isabelle T. Tran,
- Veronica Carta,
- Timothy A. Su,
- Volume 61Issue 39Angewandte Chemie International Edition
- First Published online: September 19, 2022
Timothy C. Siu
Department of Chemistry, University of California, Riverside, CA 92521 USA
Search for more papers by this authorM. Imex Aguirre Cardenas
Department of Chemistry, University of California, Riverside, CA 92521 USA
Search for more papers by this authorJacob Seo
Department of Chemistry, University of California, Riverside, CA 92521 USA
Search for more papers by this authorKirllos Boctor
Department of Chemistry, University of California, Riverside, CA 92521 USA
Search for more papers by this authorMiku G. Shimono
Department of Chemistry, University of California, Riverside, CA 92521 USA
Search for more papers by this authorIsabelle T. Tran
Department of Chemistry, University of California, Riverside, CA 92521 USA
Search for more papers by this authorDr. Veronica Carta
Department of Chemistry, University of California, Riverside, CA 92521 USA
Search for more papers by this authorCorresponding Author
Prof. Timothy A. Su
Department of Chemistry, University of California, Riverside, CA 92521 USA
Materials Science and Engineering Program, University of California, Riverside, CA 92521 USA
Search for more papers by this authorTimothy C. Siu
Department of Chemistry, University of California, Riverside, CA 92521 USA
Search for more papers by this authorM. Imex Aguirre Cardenas
Department of Chemistry, University of California, Riverside, CA 92521 USA
Search for more papers by this authorJacob Seo
Department of Chemistry, University of California, Riverside, CA 92521 USA
Search for more papers by this authorKirllos Boctor
Department of Chemistry, University of California, Riverside, CA 92521 USA
Search for more papers by this authorMiku G. Shimono
Department of Chemistry, University of California, Riverside, CA 92521 USA
Search for more papers by this authorIsabelle T. Tran
Department of Chemistry, University of California, Riverside, CA 92521 USA
Search for more papers by this authorDr. Veronica Carta
Department of Chemistry, University of California, Riverside, CA 92521 USA
Search for more papers by this authorCorresponding Author
Prof. Timothy A. Su
Department of Chemistry, University of California, Riverside, CA 92521 USA
Materials Science and Engineering Program, University of California, Riverside, CA 92521 USA
Search for more papers by this authorGraphical Abstract
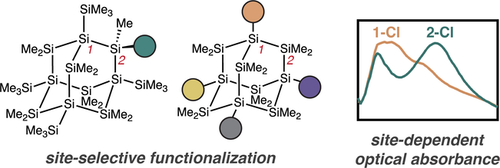
Mechanistic insight into the isomerization synthesis of sila-adamantane enables the regioselective functionalization of sila-adamantane at its 1-, 2-, 3-, 5-, and 7-positions. Substitution at the 1-position of the cluster core significantly impacts optical absorbance relative to exocyclic or 2-substitution.
Abstract
The first syntheses of functionalized sila-adamantanes via site-selective reactions are described. Mechanistic inquiry into the isomerization of sila-adamantane revealed new approaches for installing halides at the 2-position of the cluster. Meanwhile, isomerization via Lewis acid catalysts with non-nucleophilic counteranions provided access to sila-adamantane on the gram-scale, enabling us to discover strategies for substituting its 1-, 3-, 5-, and 7-positions with identical or distinct functional groups. Optical absorbance and density functional theory studies show that σ-withdrawing substituents at the 1-position strongly perturb optical absorbance in sila-adamantane, whereas substituents at the exocyclic and 2-position are optically inert. As silicon diamondoids are atomically precise models for silicon nanocrystals, our findings suggest that passivation at tertiary surface sites carries an outsized impact on the optical properties of surface-functionalized Si nanocrystals.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202206877-sup-0001-misc_information.pdf7.9 MB | Supporting Information |
| anie202206877-sup-0001-mo_ts002ts_0m_a.cif2.4 MB | Supporting Information |
| anie202206877-sup-0001-mo_ts2ts_0m.cif5.7 MB | Supporting Information |
| anie202206877-sup-0001-mo_ts3ts_0m_a.cif7.8 MB | Supporting Information |
| anie202206877-sup-0001-mo_ts4ts_0m.cif4.1 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Y. Heider, D. Scheschkewitz, Chem. Rev. 2021, 121, 9674–9718.
- 2A. Sekiguchi, T. Yatabe, S. Doi, H. Sakurai, Phosphorus Sulfur Silicon Relat. Elem. 1994, 93, 193–196.
- 3A. Sekiguchi, H. Sakurai, Adv. Organomet. Chem. 1995, 37, 1–38.
- 4E. Hengge, H. Stüger in The Chemistry of Organic Silicon Compounds, Vol. 2 (Eds.: Z. Rappoport, Y. Apeloig), Wiley, Chichester, 1998, pp. 2177–2216.
10.1002/0470857250.ch37 Google Scholar
- 5Y. Heider, D. Scheschkewitz, Dalton Trans. 2018, 47, 7104–7112.
- 6S. C. Sevov, J. M. Goicoechea, Organometallics 2006, 25, 5678–5692.
- 7D. Scheschkewitz, Angew. Chem. Int. Ed. 2005, 44, 2954–2956; Angew. Chem. 2005, 117, 3014–3016.
- 8J. M. Buriak, Chem. Rev. 2002, 102, 1271–1308.
- 9E. A. Marro, R. S. Klausen, Chem. Mater. 2019, 31, 2202–2211.
- 10J. Fischer, J. Baumgartner, C. Marschner, Science 2005, 310, 825.
- 11P. von R. Schleyer, J. Am. Chem. Soc. 1957, 79, 3292–3292.
- 12G. Fritz, F. Diem, H. Köhler, D. Kummer, H. Scheer, Angew. Chem. Int. Ed. Engl. 1970, 9, 464–465; Angew. Chem. 1970, 82, 445–446.
- 13B. Köstler, M. Bolte, H. Lerner, M. Wagner, Chem. Eur. J. 2021, 27, 14401–14404.
- 14P. von R. Schleyer, R. D. Nicholas, J. Am. Chem. Soc. 1961, 83, 182–187.
- 15S. Landa, S. Kriebel, E. Knobloch, Chem. Listy 1954, 48, 61–64.
- 16H. Stetter, M. Schwarz, A. Hirschhorn, Chem. Ber. 1959, 92, 1629–1635.
- 17H. Stetter, C. Wulff, Chem. Ber. 1960, 93, 1366–1371.
- 18L. Wanka, K. Iqbal, P. R. Schreiner, Chem. Rev. 2013, 113, 3516–3604.
- 19O. Green, T. Eilon, N. Hananya, S. Gutkin, C. R. Bauer, D. Shabat, ACS Cent. Sci. 2017, 3, 349–358.
- 20A. T. Aron, M. O. Loehr, J. Bogena, C. J. Chang, J. Am. Chem. Soc. 2016, 138, 14338–14346.
- 21L. Chen, P. Ren, B. P. Carrow, J. Am. Chem. Soc. 2016, 138, 6392–6395.
- 22K. A. Agnew-Francis, C. M. Williams, Adv. Synth. Catal. 2016, 358, 675–700.
- 23P. J. Krommenhoek, J. Wang, N. Hentz, A. C. Johnston-Peck, K. A. Kozek, G. Kalyuzhny, J. B. Tracy, ACS Nano 2012, 6, 4903–4911.
- 24B. Chen, M. Eddaoudi, T. M. Reineke, J. W. Kampf, M. O'Keeffe, O. M. Yaghi, J. Am. Chem. Soc. 2000, 122, 11559–11560.
- 25J. Tillmann, J. H. Wender, U. Bahr, M. Bolte, H.-W. Lerner, M. C. Holthausen, M. Wagner, Angew. Chem. Int. Ed. 2015, 54, 5429–5433; Angew. Chem. 2015, 127, 5519–5523.
- 26M. Bamberg, M. Bursch, A. Hansen, M. Brandl, G. Sentis, L. Kunze, M. Bolte, H.-W. Lerner, S. Grimme, M. Wagner, J. Am. Chem. Soc. 2021, 143, 10865–10871.
- 27T. Iwamoto, D. Tsushima, E. Kwon, S. Ishida, H. Isobe, Angew. Chem. Int. Ed. 2012, 51, 2340–2344; Angew. Chem. 2012, 124, 2390–2394.
- 28Y. Yokouchi, T. Iwamoto, Organometallics 2020, 39, 3301–3305.
- 29M. H. Garner, H. Li, Y. Chen, T. A. Su, Z. Shangguan, D. W. Paley, T. Liu, F. Ng, H. Li, S. Xiao, C. Nuckolls, L. Venkataraman, G. C. Solomon, Nature 2018, 558, 415–419.
- 30T. C. Siu, J. Y. Wong, M. O. Hight, T. A. Su, Phys. Chem. Chem. Phys. 2021, 23, 9643–9659.
- 31M. Zirngast, J. Baumgartner, C. Marschner, Organometallics 2008, 27, 6472–6478.
- 32A. Wallner, R. Emanuelsson, J. Baumgartner, C. Marschner, H. Ottosson, Organometallics 2013, 32, 396–405.
- 33Y. Heider, N. E. Poitiers, P. Willmes, K. I. Leszczyńska, V. Huch, D. Scheschkewitz, Chem. Sci. 2019, 10, 4523–4530.
- 34Y. Heider, P. Willmes, V. Huch, M. Zimmer, D. Scheschkewitz, J. Am. Chem. Soc. 2019, 141, 19498–19504.
- 35P. Willmes, K. Leszczyńska, Y. Heider, K. Abersfelder, M. Zimmer, V. Huch, D. Scheschkewitz, Angew. Chem. Int. Ed. 2016, 55, 2907–2910; Angew. Chem. 2016, 128, 2959–2963.
- 36L. J. Schiegerl, A. J. Karttunen, W. Klein, T. F. Fässler, Chem. Sci. 2019, 10, 9130–9139.
- 37S. Joseph, M. Hamberger, F. Mutzbauer, O. Härtl, M. Meier, N. Korber, Angew. Chem. Int. Ed. 2009, 48, 8770–8772; Angew. Chem. 2009, 121, 8926–8929.
- 38M. Waibel, F. Kraus, S. Scharfe, B. Wahl, T. F. Fässler, Angew. Chem. Int. Ed. 2010, 49, 6611–6615; Angew. Chem. 2010, 122, 6761–6765.
- 39F. S. Geitner, T. F. Fässler, Chem. Commun. 2017, 53, 12974–12977.
- 40H. Gilman, C. L. Smith, J. Organomet. Chem. 1967, 8, 245–253.
- 41S. M. Whittaker, M.-C. Brun, F. Cervantes-Lee, K. H. Pannell, J. Organomet. Chem. 1995, 499, 247–252.
- 42C. Kayser, G. Kickelbick, C. Marschner, Angew. Chem. Int. Ed. 2002, 41, 989–992;
10.1002/1521-3773(20020315)41:6<989::AID-ANIE989>3.0.CO;2-A CAS PubMed Web of Science® Google ScholarAngew. Chem. 2002, 114, 1031–1034.
- 43R. Fischer, T. Konopa, S. Ully, J. Baumgartner, C. Marschner, J. Organomet. Chem. 2003, 685, 79–92.
- 44L. Albers, J. Baumgartner, C. Marschner, T. Müller, Chem. Eur. J. 2016, 22, 7970–7977.
- 45Deposition Numbers 2143761, 2143763, 2143760, and 2143762 contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 46M. Ishikawa, J. Iyoda, H. Ikeda, K. Kotake, T. Hashimoto, M. Kumada, J. Am. Chem. Soc. 1981, 103, 4845–4850.
- 47S. Sharma, N. Caballero, H. Li, K. H. Pannell, Organometallics 1999, 18, 2855–2860.
- 48M. Ishikawa, M. Watanabe, J. Iyoda, H. Ikeda, M. Kumada, Organometallics 1982, 1, 317–322.
- 49T. A. Blinka, R. West, Organometallics 1986, 5, 128–133.
- 50H. Wagner, A. Wallner, J. Fischer, M. Flock, J. Baumgartner, C. Marschner, Organometallics 2007, 26, 6704–6717.
- 51H. Wagner, J. Baumgartner, C. Marschner, P. Poelt, Organometallics 2011, 30, 3939–3954.
- 52H. Wagner, J. Baumgartner, T. Müller, C. Marschner, J. Am. Chem. Soc. 2009, 131, 5022–5023.
- 53L. Albers, S. Rathjen, J. Baumgartner, C. Marschner, T. Müller, J. Am. Chem. Soc. 2016, 138, 6886–6892.
- 54L. Albers, M. A. Meshgi, J. Baumgartner, C. Marschner, T. Müller, Organometallics 2015, 34, 3756–3763.
- 55H. F. T. Klare, L. Albers, L. Süsse, S. Keess, T. Müller, M. Oestreich, Chem. Rev. 2021, 121, 5889–5985.
- 56E. M. Engler, M. Farcasiu, A. Sevin, J. M. Cense, P. von R. Schleyer, J. Am. Chem. Soc. 1973, 95, 5769–5771.
- 57M. Ishikawa, M. Kumada, H. Sakurai, J. Organomet. Chem. 1970, 23, 63–69.
- 58C. Marschner, Eur. J. Inorg. Chem. 1998, 221–226.
10.1002/(SICI)1099-0682(199802)1998:2<221::AID-EJIC221>3.0.CO;2-G CAS Web of Science® Google Scholar
- 59C. Marschner, in Organosilicon Compounds (Ed.: V. Ya. Lee), Elsevier, Amsterdam, 2017, pp. 295–360.
10.1016/B978-0-12-801981-8.00007-1 Google Scholar
- 60J. Bedard, N. J. Roberts, M. Shayan, K. L. Bamford, U. Werner-Zwanziger, K. M. Marczenko, S. S. Chitnis, Angew. Chem. Int. Ed. 2022, 61, e202204851; Angew. Chem. 2022, 134, e202204851.
- 61V. Humblet, P. Misra, K. R. Bhushan, K. Nasr, Y. Sen Ko, T. Tsukamoto, N. Pannier, J. V. Frangioni, W. Maison, J. Med. Chem. 2009, 52, 544–550.
- 62G. R. Newkome, A. Nayak, R. K. Behera, C. N. Moorefield, G. R. Baker, J. Org. Chem. 1992, 57, 358–362.
- 63W. L. Wilson, P. F. Szajowski, L. E. Brus, Science 1993, 262, 1242–1244.
- 64M. V. Wolkin, J. Jorne, P. M. Fauchet, G. Allan, C. Delerue, Phys. Rev. Lett. 1999, 82, 197–200.
- 65Q. Li, Y. He, J. Chang, L. Wang, H. Chen, Y. W. Tan, H. Wang, Z. Shao, J. Am. Chem. Soc. 2013, 135, 14924–14927.
- 66R. K. Baldwin, K. A. Pettigrew, E. Ratai, M. P. Augustine, S. M. Kauzlarich, Chem. Commun. 2002, 1822–1823.
- 67L. M. Wheeler, N. R. Neale, T. Chen, U. R. Kortshagen, Nat. Commun. 2013, 4, 2197.
- 68M. Dasog, J. Kehrle, B. Rieger, J. G. C. Veinot, Angew. Chem. Int. Ed. 2016, 55, 2322–2339; Angew. Chem. 2016, 128, 2366–2384.
- 69I. Vasiliev, J. R. Chelikowsky, R. M. Martin, Phys. Rev. B 2002, 65, 121302.
- 70Z. Zhou, L. Brus, R. Friesner, Nano Lett. 2003, 3, 163–167.
- 71Z. Zhou, R. A. Friesner, L. Brus, J. Am. Chem. Soc. 2003, 125, 15599–15607.
- 72A. Martínez, J. C. Alonso, L. E. Sansores, R. Salcedo, J. Phys. Chem. C 2010, 114, 12427–12431.
- 73E. Ramos, B. M. Monroy, J. C. Alonso, L. E. Sansores, R. Salcedo, A. Martínez, J. Phys. Chem. C 2012, 116, 3988–3994.
- 74M. H. Garner, H. Li, M. Neupane, Q. Zou, T. Liu, T. A. Su, Z. Shangguan, D. W. Paley, F. Ng, S. Xiao, C. Nuckolls, L. Venkataraman, G. C. Solomon, J. Am. Chem. Soc. 2019, 141, 15471–15476.
- 75S. Gunasekaran, J. E. Greenwald, L. Venkataraman, Nano Lett. 2020, 20, 2843–2848.
- 76F. Evers, R. Korytár, S. Tewari, J. M. Van Ruitenbeek, Rev. Mod. Phys. 2020, 92, 35001.