Ruthenium-Catalyzed Geminal Hydroborative Cyclization of Enynes
Dr. Yun-Xuan Tan
Department of Chemistry and the Hong Kong Branch of Chinese National Engineering Research Centre for Tissue Restoration & Reconstruction, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong SAR, China
Search for more papers by this authorShijia Li
Department of Chemistry and the Hong Kong Branch of Chinese National Engineering Research Centre for Tissue Restoration & Reconstruction, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong SAR, China
Shenzhen Bay Laboratory, Shenzhen, 518055 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Lijuan Song
School of Science, Harbin Institute of Technology (Shenzhen), Shenzhen, 518055 China
Search for more papers by this authorProf. Dr. Xinhao Zhang
Shenzhen Bay Laboratory, Shenzhen, 518055 China
Lab of Computational Chemistry and Drug Design, State Key Laboratory of Chemical Oncogenomics, Peking University Shenzhen Graduate School, Shenzhen, 518055 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Yun-Dong Wu
Shenzhen Bay Laboratory, Shenzhen, 518055 China
Lab of Computational Chemistry and Drug Design, State Key Laboratory of Chemical Oncogenomics, Peking University Shenzhen Graduate School, Shenzhen, 518055 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Jianwei Sun
Department of Chemistry and the Hong Kong Branch of Chinese National Engineering Research Centre for Tissue Restoration & Reconstruction, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong SAR, China
Search for more papers by this authorDr. Yun-Xuan Tan
Department of Chemistry and the Hong Kong Branch of Chinese National Engineering Research Centre for Tissue Restoration & Reconstruction, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong SAR, China
Search for more papers by this authorShijia Li
Department of Chemistry and the Hong Kong Branch of Chinese National Engineering Research Centre for Tissue Restoration & Reconstruction, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong SAR, China
Shenzhen Bay Laboratory, Shenzhen, 518055 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Lijuan Song
School of Science, Harbin Institute of Technology (Shenzhen), Shenzhen, 518055 China
Search for more papers by this authorProf. Dr. Xinhao Zhang
Shenzhen Bay Laboratory, Shenzhen, 518055 China
Lab of Computational Chemistry and Drug Design, State Key Laboratory of Chemical Oncogenomics, Peking University Shenzhen Graduate School, Shenzhen, 518055 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Yun-Dong Wu
Shenzhen Bay Laboratory, Shenzhen, 518055 China
Lab of Computational Chemistry and Drug Design, State Key Laboratory of Chemical Oncogenomics, Peking University Shenzhen Graduate School, Shenzhen, 518055 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Jianwei Sun
Department of Chemistry and the Hong Kong Branch of Chinese National Engineering Research Centre for Tissue Restoration & Reconstruction, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong SAR, China
Search for more papers by this authorGraphical Abstract
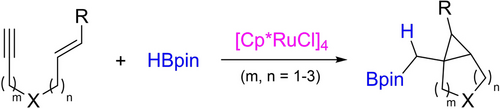
Different from the previously known hydroborative cyclizations that add hydrogen and boron to opposite sides of an enyne, the proper choice of a ruthenium catalyst alters this propensity to a new addition mode, geminal hydroborative cyclopropanation. Two possible mechanisms are operative, which are substrate-dependent based on DFT studies.
Abstract
Disclosed here is the first geminal (gem-) hydroborative cyclization of enynes. Different from known hydroborative cyclizations, this process adds hydrogen and boron to the same position, leading to a new reaction mode. With [Cp*RuCl]4 as catalyst, a range of gem-hydroborated bicyclic products bearing a cyclopropane unit could be rapidly assembled from simple enyne substrates. Control experiments and density functional theory (DFT) calculations provided important insights into the reaction mechanism. Notably, two major competing pathways may operate with substrate-dependence. 1,6-Enynes favor initial oxidative cyclometalation to form a ruthenacyclopentene intermediate prior to engaging hydroborane, while other enynes (e.g., 1,7-enynes) that lack strong propensity toward cyclization prefer initial alkyne gem-(H,B)-addition to form an α-boryl ruthenium carbene followed by intramolecular olefin cyclopropanation. This process also represents the first ruthenium-catalyzed enyne hydroborative cyclization.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202204319-sup-0001-CCDC2129409.cif268.9 KB | Supporting Information |
| anie202204319-sup-0001-misc_information.pdf10.7 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Reviews of alkyne hydroboration:
- 1aH. Yoshida, ACS Catal. 2016, 6, 1799–1811;
- 1bJ. Carreras, A. Caballero, P. J. Pérez, Chem. Asian J. 2019, 14, 329–343;
- 1cJ. Guo, Z. Cheng, J. Chen, X. Chen, Z. Lu, Acc. Chem. Res. 2021, 54, 2701–2716.
- 2For recent reviews on the utility of organoboranes:
- 2aD. G. Hall, J. C. H. Lee, J. Ding, Pure Appl. Chem. 2012, 84, 2263–2277;
- 2bA. J. Lennox, G. C. Lloyd-Jones, Chem. Soc. Rev. 2014, 43, 412–443;
- 2cL. Xu, S. Zhang, P. Li, Chem. Soc. Rev. 2015, 44, 8848–8858.
- 3Enyne cyclization reviews:
- 3aC. Aubert, O. Buisine, M. Malacria, Chem. Rev. 2002, 102, 813–834;
- 3bL. Zhang, J. Sun, S. A. Kozmin, Adv. Synth. Catal. 2006, 348, 2271–2296;
- 3cA. Fürstner, Chem. Soc. Rev. 2009, 38, 3208–3221;
- 3dR. Dorel, A. M. Echavarren, Chem. Rev. 2015, 115, 9028–9072;
- 3eC. I. Stathakis, P. L. Gkizis, A. L. Zografos, Nat. Prod. Rep. 2016, 33, 1093–1117;
- 3fY. Hu, M. Bai, Y. Yang, Q. Zhou, Org. Chem. Front. 2017, 4, 2256–2275.
- 4Reviews of hydroborative enynes cyclization:
- 4aE. Buñuel, D. J. Cárdenas, Eur. J. Org. Chem. 2016, 5446–5464;
- 4bE. Buñuel, D. J. Cárdenas, Chem. Eur. J. 2018, 24, 11239–11244;
- 4cL. Mao, S. K. Bose, Adv. Synth. Catal. 2020, 362, 4174–4188.
- 5For recent examples on hydroborative cyclization of enynes:
- 5aR. E. Kinder, R. A. Widenhoefer, Org. Lett. 2006, 8, 1967–1969;
- 5bJ. Marco-Martínez, V. Lopez-Carrillo, E. Buñuel, R. Simancas, D. J. Cárdenas, J. Am. Chem. Soc. 2007, 129, 1874–1875;
- 5cV. Pardo-Rodríguez, E. Buñuel, D. Collado-Sanz, D. J. Cárdenas, Chem. Commun. 2012, 48, 10517–10519;
- 5dP. Liu, Y. Fukui, P. Tian, Z.-T. He, C.-Y. Sun, N.-Y. Wu, G.-Q. Lin, J. Am. Chem. Soc. 2013, 135, 11700–11703;
- 5eT. Xi, Z. Lu, ACS Catal. 2017, 7, 1181–1185;
- 5fS. Yu, C. Wu, S. Ge, J. Am. Chem. Soc. 2017, 139, 6526–6529;
- 5gC. Wang, S. Ge, J. Am. Chem. Soc. 2018, 140, 10687–10690;
- 5hN. Cabrera-Lobera, P. Rodriguez-Salamanca, J. C. Nieto-Carmona, E. Buñuel, D. J. Cárdenas, Chem. Eur. J. 2018, 24, 784–788;
- 5iN. Cabrera-Lobera, M. T. Quirós, E. Buñuel, D. J. Cárdenas, Catal. Sci. Technol. 2019, 9, 1021–1029;
- 5jC. Wu, J. Liao, S. Ge, Angew. Chem. Int. Ed. 2019, 58, 8882–8886; Angew. Chem. 2019, 131, 8974–8978.
- 6M. M. Hansmann, R. L. Melen, M. Rudolph, F. Rominger, H. Wadepohl, D. W. Stephan, A. S. K. Hashmi, J. Am. Chem. Soc. 2015, 137, 15469–15477.
- 7Q. Feng, H. Wu, X. Li, L. Song, L. W. Chung, Y.-D. Wu, J. Sun, J. Am. Chem. Soc. 2020, 142, 13867–13877.
- 8For other mechanistically related ruthenium-catalyzed gem-additions:
- 8aK. Radkowski, B. Sundararaju, A. Fürstner, Angew. Chem. Int. Ed. 2013, 52, 355–360; Angew. Chem. 2013, 125, 373–378;
- 8bM. Leutzsch, L. M. Wolf, P. Gupta, M. Fuchs, W. Thiel, C. Farès, A. Fürstner, Angew. Chem. Int. Ed. 2015, 54, 12431–12436; Angew. Chem. 2015, 127, 12608–12613;
- 8cA. Guthertz, M. Leutzsch, L. M. Wolf, P. Gupta, S. M. Rummelt, R. Goddard, C. Farès, W. Thiel, A. Fürstner, J. Am. Chem. Soc. 2018, 140, 3156–3169;
- 8dT. Biberger, C. P. Gordon, M. Leutzsch, S. Peil, A. Guthertz, C. Copéret, A. Fürstner, Angew. Chem. Int. Ed. 2019, 58, 8845–8850; Angew. Chem. 2019, 131, 8937–8942;
- 8eA. Fürstner, J. Am. Chem. Soc. 2019, 141, 11–24;
- 8fS. Peil, A. Guthertz, T. Biberger, A. Fürstner, Angew. Chem. Int. Ed. 2019, 58, 8851–8856; Angew. Chem. 2019, 131, 8943–8948;
- 8gS. Peil, A. Fürstner, Angew. Chem. Int. Ed. 2019, 58, 18476–18481; Angew. Chem. 2019, 131, 18647–18652;
- 8hL. Song, Q. Feng, Y. Wang, S. Ding, Y.-D. Wu, X. Zhang, L. W. Chung, J. Sun, J. Am. Chem. Soc. 2019, 141, 17441–17451;
- 8iT. Biberger, R. J. Zachmann, A. Fürstner, Angew. Chem. Int. Ed. 2020, 59, 18423–18429; Angew. Chem. 2020, 132, 18581–18587;
- 8jS. Peil, G. Bistoni, R. Goddard, A. Fürstner, J. Am. Chem. Soc. 2020, 142, 18541–18553;
- 8kR. J. Zachmann, A. Fürstner, Chem. Eur. J. 2021, 27, 7663–7666;
- 8lT. Biberger, S. N. Hess, M. Leutzsch, A. Fürstner, Angew. Chem. Int. Ed. 2022, 61, e202113827; Angew. Chem. 2022, 134, e202113827;
- 8mS. Peil, A. G. González, M. Leutzsch, A. Fürstner, J. Am. Chem. Soc. 2022, 144, 4158–4167.
- 9
- 9aC. Gunanathan, M. Holscher, F. Pan, W. Leitner, J. Am. Chem. Soc. 2012, 134, 14349–14352;
- 9bB. Sundararaju, A. Fürstner, Angew. Chem. Int. Ed. 2013, 52, 14050–14054; Angew. Chem. 2013, 125, 14300–14304;
- 9cL.-J. Song, T. Wang, X. Zhang, L. W. Chung, Y.-D. Wu, ACS Catal. 2017, 7, 1361–1368;
- 9dK. Yamamoto, Y. Mohara, Y. Mutoh, S. Saito, J. Am. Chem. Soc. 2019, 141, 17042–17047.
- 10Selected other examples:
- 10aB. M. Trost, Z. T. Ball, J. Am. Chem. Soc. 2001, 123, 12726–12727;
- 10bB. M. Trost, Z. T. Ball, J. Am. Chem. Soc. 2005, 127, 17644–17655;
- 10cS. Ding, L.-J. Song, L. W. Chung, X. Zhang, J. Sun, Y.-D. Wu, J. Am. Chem. Soc. 2013, 135, 13835–13842;
- 10dS. M. Rummelt, A. Fürstner, Angew. Chem. Int. Ed. 2014, 53, 3626–3630; Angew. Chem. 2014, 126, 3700–3704;
- 10eS. Ding, L.-J. Song, Y. Wang, X. Zhang, L. W. Chung, Y.-D. Wu, J. Sun, Angew. Chem. Int. Ed. 2015, 54, 5632–5635; Angew. Chem. 2015, 127, 5724–5727.
- 11
- 11aD. S. Kania, J. D. Gonzalvo, Z. A. Weber, Clin. Ther. 2011, 33, 1005–1022;
- 11bS. J. Keam, A. J. Wagstaff, Treat. Endocrinol. 2003, 2, 49–70.
- 12Deposition Number 2129409 contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 13S. W. Roh, K. Choi, C. Lee, Chem. Rev. 2019, 119, 4293–4356.
- 14The formation of bicyclo[3.1.0]hexanes catalyzed by ruthenium:
- 14aF. Monnier, D. Castillo, S. Dérien, L. Toupet, P. H. Dixneuf, Angew. Chem. Int. Ed. 2003, 42, 5474–5477; Angew. Chem. 2003, 115, 5632–5635;
- 14bF. Monnier, C. Vovard-Le Bray, D. Castillo, V. Aubert, S. Dérien, P. H. Dixneuf, L. Toupet, A. Ienco, C. Mealli, J. Am. Chem. Soc. 2007, 129, 6037–6049;
- 14cB. M. Trost, A. Breder, B. M. O'Keefe, M. Rao, A. W. Franz, J. Am. Chem. Soc. 2011, 133, 4766–4769;
- 14dB. M. Trost, M. C. Ryan, M. Rao, T. Z. Markovic, J. Am. Chem. Soc. 2014, 136, 17422–17425;
- 14eB. M. Trost, M. C. Ryan, M. Rao, Beilstein J. Org. Chem. 2016, 12, 1136–1152;
- 14fM. Gao, Q. Gao, X. Hao, Y. Wu, Q. Zhang, G. Liu, R. Liu, Org. Lett. 2020, 22, 1139–1143.
- 15
- 15aN. Saito, D. Tanaka, M. Mori, Y. Sato, Chem. Rec. 2011, 11, 186–198;
- 15bS. M. Rummelt, G.-J. Cheng, P. Gupta, W. Thiel, A. Fürstner, Angew. Chem. Int. Ed. 2017, 56, 3599–3604; Angew. Chem. 2017, 129, 3653–3658.
- 16
- 16aN. Chatani, K. Kataoka, S. Murai, N. Furukawa, Y. Seki, J. Am. Chem. Soc. 1998, 120, 9104–9105;
- 16bD. Tanaka, Y. Sato, M. Mori, J. Am. Chem. Soc. 2007, 129, 7730–7731.
- 16cWe also attempted to trap the carbene intermediate B-int3 using an additional intramolecular alkene attached to the existing alkene motif. Unfortunately, no desired cyclopropanation product was detected.
- 17
- 17aB. Martín-Matute, C. Nevado, D. J. Cárdenas, A. M. Echavarren, J. Am. Chem. Soc. 2003, 125, 5757–5766;
- 17bC. Bruneau, Angew. Chem. Int. Ed. 2005, 44, 2328–2334; Angew. Chem. 2005, 117, 2380–2386;
- 17cC. Nieto-Oberhuber, M. P. Muñoz, S. López, E. Jiménez-Núñez, C. Nevado, E. Herrero-Gómez, M. Raducan, A. M. Echavarren, Chem. Eur. J. 2006, 12, 1677–1693;
- 17dA. Fürstner, C. C. Stimson, Angew. Chem. Int. Ed. 2007, 46, 8845–8849; Angew. Chem. 2007, 119, 9001–9005;
- 17eK. Ota, S. I. Lee, J.-M. Tang, M. Takachi, H. Nakai, T. Morimoto, H. Sakurai, K. Kataoka, N. Chatani, J. Am. Chem. Soc. 2009, 131, 15203–15211.
- 18We tried mixing a stoichiometric amount of [Cp*RuCl]4 with 1 a, but did not observe the formation of intermediate int1 by nuclear magnetic resonance (NMR) spectroscopy, even at low temperature.