Electrochemical Oxo-Fluorosulfonylation of Alkynes under Air: Facile Access to β-Keto Sulfonyl Fluorides
Dr. Dengfeng Chen
Jiangsu Co-Innovation Center of Efficient Processing and Utilization of Forest Resources, International Innovation Center for Forest Chemicals and Materials, Nanjing Forestry University, Nanjing, 210037 China
Search for more papers by this authorXingliang Nie
Key Laboratory of Molecule Synthesis and Function Discovery (Fujian Province University), College of Chemistry, Fuzhou University, Fuzhou, 350108 China
Search for more papers by this authorQingyuan Feng
Jiangsu Co-Innovation Center of Efficient Processing and Utilization of Forest Resources, International Innovation Center for Forest Chemicals and Materials, Nanjing Forestry University, Nanjing, 210037 China
Search for more papers by this authorYingyin Zhang
Jiangsu Co-Innovation Center of Efficient Processing and Utilization of Forest Resources, International Innovation Center for Forest Chemicals and Materials, Nanjing Forestry University, Nanjing, 210037 China
Search for more papers by this authorYiheng Wang
Co-Innovation Center for Sustainable Forestry in Southern China, Nanjing Forestry University, Nanjing, 210037 China
Search for more papers by this authorQiuyue Wang
Co-Innovation Center for Sustainable Forestry in Southern China, Nanjing Forestry University, Nanjing, 210037 China
Search for more papers by this authorProf. Dr. Lin Huang
Co-Innovation Center for Sustainable Forestry in Southern China, Nanjing Forestry University, Nanjing, 210037 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Shenlin Huang
Jiangsu Co-Innovation Center of Efficient Processing and Utilization of Forest Resources, International Innovation Center for Forest Chemicals and Materials, Nanjing Forestry University, Nanjing, 210037 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Saihu Liao
Key Laboratory of Molecule Synthesis and Function Discovery (Fujian Province University), College of Chemistry, Fuzhou University, Fuzhou, 350108 China
Beijing National Laboratory for Molecular Sciences, Beijing, 100190 China
Search for more papers by this authorDr. Dengfeng Chen
Jiangsu Co-Innovation Center of Efficient Processing and Utilization of Forest Resources, International Innovation Center for Forest Chemicals and Materials, Nanjing Forestry University, Nanjing, 210037 China
Search for more papers by this authorXingliang Nie
Key Laboratory of Molecule Synthesis and Function Discovery (Fujian Province University), College of Chemistry, Fuzhou University, Fuzhou, 350108 China
Search for more papers by this authorQingyuan Feng
Jiangsu Co-Innovation Center of Efficient Processing and Utilization of Forest Resources, International Innovation Center for Forest Chemicals and Materials, Nanjing Forestry University, Nanjing, 210037 China
Search for more papers by this authorYingyin Zhang
Jiangsu Co-Innovation Center of Efficient Processing and Utilization of Forest Resources, International Innovation Center for Forest Chemicals and Materials, Nanjing Forestry University, Nanjing, 210037 China
Search for more papers by this authorYiheng Wang
Co-Innovation Center for Sustainable Forestry in Southern China, Nanjing Forestry University, Nanjing, 210037 China
Search for more papers by this authorQiuyue Wang
Co-Innovation Center for Sustainable Forestry in Southern China, Nanjing Forestry University, Nanjing, 210037 China
Search for more papers by this authorProf. Dr. Lin Huang
Co-Innovation Center for Sustainable Forestry in Southern China, Nanjing Forestry University, Nanjing, 210037 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Shenlin Huang
Jiangsu Co-Innovation Center of Efficient Processing and Utilization of Forest Resources, International Innovation Center for Forest Chemicals and Materials, Nanjing Forestry University, Nanjing, 210037 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Saihu Liao
Key Laboratory of Molecule Synthesis and Function Discovery (Fujian Province University), College of Chemistry, Fuzhou University, Fuzhou, 350108 China
Beijing National Laboratory for Molecular Sciences, Beijing, 100190 China
Search for more papers by this authorGraphical Abstract
The generation of FSO2 radicals under electrochemical conditions is reported. Various sulfonyl fluorides and heterocycles, including a novel oxathiazole dioxide heterocycle, can be obtained in good to excellent yields. Some β-keto sulfonyl fluorides and derivatives showed potent activities against Bursaphelenchus xylophilus and Colletotrichum gloeosporioides.
Abstract
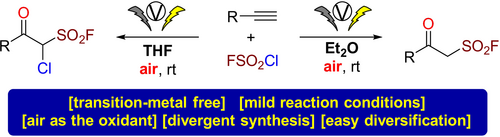
Radical fluorosulfonylation is emerging as an appealing approach for the synthesis of sulfonyl fluorides, which have widespread applications in many fields, in particular in the context of chemical biology and drug development. Here, we report the first investigation of FSO2 radical generation under electrochemical conditions, and the establishment of a new and facile approach for the synthesis of β-keto sulfonyl fluorides via oxo-fluorosulfonylation of alkynes with sulfuryl chlorofluoride as the radical precursor and air as the oxidant. This electrochemical protocol is amenable to access two different products (β-keto sulfonyl fluorides or α-chloro-β-keto sulfonyl fluorides) with the same reactants. The β-keto sulfonyl fluoride products can be utilized as useful building blocks in the synthesis of various derivatives and heterocycles, including the first synthesis of an oxathiazole dioxide compound. Furthermore, some β-keto sulfonyl fluorides and derivatives exhibited notably potent activities against Bursaphelenchus xylophilus and Colletotrichum gloeosporioides.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202112118-sup-0001-cif.zip134 KB | Supporting Information |
| anie202112118-sup-0001-misc_information.pdf22.5 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. Dong, L. Krasnova, M. G. Finn, K. B. Sharpless, Angew. Chem. Int. Ed. 2014, 53, 9430–9448; Angew. Chem. 2014, 126, 9584–9603;
- 1bP. K. Chinthakindi, P. I. Arvidsson, Eur. J. Org. Chem. 2018, 3648–3666;
- 1cA. S. Barrow, C. J. Smedley, Q. Zheng, S. Li, J. Dong, J. E. Moses, Chem. Soc. Rev. 2019, 48, 4731–4758;
- 1dL. Xu, J. Dong, Chin. J. Chem. 2020, 38, 414–419;
- 1eC. Lee, A. J. Cook, J. E. Elisabeth, N. C. Friede, G. M. Sammis, N. D. Ball, ACS Catal. 2021, 11, 6578–6589.
- 2
- 2aA. Narayanan, L. H. Jones, Chem. Sci. 2015, 6, 2650–2659;
- 2bL. H. Jones, ACS Med. Chem. Lett. 2018, 9, 584–586;
- 2cP. Martín-Gago, C. A. Olsen, Angew. Chem. Int. Ed. 2019, 58, 957–966; Angew. Chem. 2019, 131, 969–978;
- 2dL. H. Jones, J. W. Kelly, RSC Med. Chem. 2020, 11, 10–17;
- 2eS. E. Dalton, S. Campos, ChemBioChem 2020, 21, 1080–1100.
- 3
- 3aZ. Liu, J. Li, S. Li, G. Li, K. B. Sharpless, P. Wu, J. Am. Chem. Soc. 2018, 140, 2919–2925;
- 3bR. Artschwager, D. J. Ward, S. Gannon, A. J. Brouwer, H. van de Langemheen, H. Kowalski, R. M. J. Liskamp, J. Med. Chem. 2018, 61, 5395–5411;
- 3cX. Yang, T. J. M. Michiels, C. de Jong, M. Soethoudt, N. Dekker, E. Gordon, M. van der Stelt, L. H. Heitman, D. van der Es, A. P. IJzerman, J. Med. Chem. 2018, 61, 7892–7901;
- 3dC. Baggio, P. Udompholkul, L. Gambini, A. F. Salem, J. Jossart, J. J. P. Perry, M. Pellecchia, J. Med. Chem. 2019, 62, 9188–9200;
- 3eH. Xu, F. Ma, N. Wang, W. Hou, H. Xiong, F. Lu, J. Li, S. Wang, P. Ma, G. Yang, R. A. Lerner, Adv. Sci. 2019, 6, 1901551;
- 3fC. Liu, Q. Zhou, Y. Li, L. V. Garner, S. P. Watkins, L. J. Carter, J. Smoot, A. C. Gregg, A. D. Daniels, S. Jervey, D. Albaiu, ACS Cent. Sci. 2020, 6, 315–331;
- 3gQ. Li, Q. Chen, P. C. Klauser, M. Li, F. Zheng, N. Wang, X. Li, Q. Zhang, X. Fu, Q. Wang, Y. Xu, L. Wang, Cell 2020, 182, 85–97;
- 3hS. Kitamura, Q. Zheng, J. L. Woehl, A. Solania, E. Chen, N. Dillon, M. V. Hull, M. Kotaniguchi, J. R. Cappiello, S. Kitamura, V. Nizet, K. B. Sharpless, D. W. Wolan, J. Am. Chem. Soc. 2020, 142, 10899–10904;
- 3iM. Teng, S. B. Ficarro, H. Yoon, J. Che, J. Zhou, E. S. Fischer, J. A. Marto, T. Zhang, N. S. Gray, ACS Med. Chem. Lett. 2020, 11, 1269–1273.
- 4
- 4aG.-F. Zha, W.-Y. Fang, Y.-G. Li, J. Leng, X. Chen, H.-L. Qin, J. Am. Chem. Soc. 2018, 140, 17666–17673;
- 4bN. Akporji, J. Lieberman, M. Maser, M. Yoshimura, Z. Boskovic, B. H. Lipshutz, ChemCatChem 2019, 11, 5743–5747;
- 4cG. Meng, T. Guo, T. Ma, J. Zhang, Y. Shen, K. B. Sharpless, J. Dong, Nature 2019, 574, 86–89;
- 4dC. J. Smedley, Q. Zheng, B. Gao, S. Li, A. Molino, H. M. Duivenvoorden, B. S. Parker, D. J. D. Wilson, K. B. Sharpless, J. E. Moses, Angew. Chem. Int. Ed. 2019, 58, 4552–4556; Angew. Chem. 2019, 131, 4600–4604;
- 4eP. J. Foth, F. Gu, T. G. Bolduc, S. S. Kanani, G. M. Sammis, Chem. Sci. 2019, 10, 10331–10335;
- 4fM.-C. Giel, C. J. Smedley, E. R. R. Mackie, T. Guo, J. Dong, T. P. Soares da Costa, J. E. Moses, Angew. Chem. Int. Ed. 2020, 59, 1181–1186; Angew. Chem. 2020, 132, 1197–1202;
- 4gM. Mendel, I. Kalvet, D. Hupperich, G. Magnin, F. Schoenebeck, Angew. Chem. Int. Ed. 2020, 59, 2115–2119; Angew. Chem. 2020, 132, 2132–2136;
- 4hD.-D. Liang, D. E. Streefkerk, D. Jordaan, J. Wagemakers, J. Baggerman, H. Zuilhof, Angew. Chem. Int. Ed. 2020, 59, 7494–7500; Angew. Chem. 2020, 132, 7564–7570;
- 4iS. Mahapatra, C. P. Woroch, T. W. Butler, S. N. Carneiro, S. C. Kwan, S. R. Khasnavis, J. Gu, J. K. Dutra, B. C. Vetelino, J. Bellenger, C. W. am Ende, N. D. Ball, Org. Lett. 2020, 22, 4389–4394.
- 5
- 5aJ. Dong, K. B. Sharpless, L. Kwisnek, J. S. Oakdale, V. V. Fokin, Angew. Chem. Int. Ed. 2014, 53, 9466–9470; Angew. Chem. 2014, 126, 9620–9624;
- 5bB. Gao, L. Zhang, Q. Zheng, F. Zhou, L. M. Klivansky, J. Lu, Y. Liu, J. Dong, P. Wu, K. B. Sharpless, Nat. Chem. 2017, 9, 1083–1088;
- 5cT. Hmissa, X. Zhang, N. R. Dhumal, G. J. McManus, X. Zhou, H. B. Nulwala, A. Mirjafari, Angew. Chem. Int. Ed. 2018, 57, 16005–16009; Angew. Chem. 2018, 130, 16237–16241;
- 5dC. Yang, J. P. Flynn, J. Niu, Angew. Chem. Int. Ed. 2018, 57, 16194–16199; Angew. Chem. 2018, 130, 16426–16431;
- 5eK. Durie, J. Yatvin, M. Kovaliov, G. H. Crane, J. Horn, S. Averick, J. Locklin, Macromolecules 2018, 51, 297–305;
- 5fH. Wan, S. Zhou, P. Gu, F. Zhou, D. Lyu, Q. Xu, A. Wang, H. Shi, Q. Xu, J. Lu, Polym. Chem. 2020, 11, 1033–1042;
- 5gZ. Cao, F. Zhou, P.-Y. Gu, D. Chen, J. He, J. R. Cappiello, P. Wu, Q. Xu, J. Lu, Polym. Chem. 2020, 11, 3120–3124.
- 6
- 6aA. T. Davies, J. M. Curto, S. W. Bagley, M. C. Willis, Chem. Sci. 2017, 8, 1233–1237;
- 6bA. L. Tribby, I. Rodríguez, S. Shariffudin, N. D. Ball, J. Org. Chem. 2017, 82, 2294–2299;
- 6cG. Laudadio, A. de A. Bartolomeu, L. M. H. M. Verwijlen, Y. Cao, K. T. de Oliveira, T. Noël, J. Am. Chem. Soc. 2019, 141, 11832–11836;
- 6dT. S.-B. Lou, S. W. Bagley, M. C. Willis, Angew. Chem. Int. Ed. 2019, 58, 18859–18863; Angew. Chem. 2019, 131, 19035–19039;
- 6eL. Wang, J. Cornella, Angew. Chem. Int. Ed. 2020, 59, 23510–23515; Angew. Chem. 2020, 132, 23716–23721;
- 6fY. Liu, D. Yu, Y. Guo, J.-C. Xiao, Q.-Y. Chen, C. Liu, Org. Lett. 2020, 22, 2281–2286;
- 6gT. Zhong, M.-K. Pang, Z.-D. Chen, B. Zhang, J. Weng, G. Lu, Org. Lett. 2020, 22, 3072–3078;
- 6hT. Xu, T. Cao, M. Yang, R. Xu, X. Nie, S. Liao, Org. Lett. 2020, 22, 3692–3696;
- 6iM. Pérez-Palau, J. Cornella, Eur. J. Org. Chem. 2020, 2497–2500;
- 6jS. Liu, Y. Huang, X.-H. Xu, F.-L. Qing, J. Fluorine Chem. 2020, 240, 109653;
- 6kT. Zhong, J.-T. Yi, Z.-D. Chen, Q.-C. Zhuang, Y.-Z. Li, G. Lu, J. Weng, Chem. Sci. 2021, 12, 9359–9365.
- 7
- 7aT. Guo, G. Meng, X. Zhan, Q. Yang, T. Ma, L. Xu, K. B. Sharpless, J. Dong, Angew. Chem. Int. Ed. 2018, 57, 2605–2610; Angew. Chem. 2018, 130, 2635–2640;
- 7bH. Zhou, P. Mukherjee, R. Liu, E. Evrard, D. Wang, J. M. Humphrey, T. W. Butler, L. R. Hoth, J. B. Sperry, S. K. Sakata, C. J. Helal, C. W. am Ende, Org. Lett. 2018, 20, 812–815;
- 7cJ. Kwon, B. M. Kim, Org. Lett. 2019, 21, 428–433;
- 7dC. Lee, N. D. Ball, G. M. Sammis, Chem. Commun. 2019, 55, 14753–14756.
- 8
- 8aX. Nie, T. Xu, J. Song, A. Devaraj, B. Zhang, Y. Chen, S. Liao, Angew. Chem. Int. Ed. 2021, 60, 3956–3960; Angew. Chem. 2021, 133, 4002–4006;
- 8bX. Nie, T. Xu, Y. Hong, H. Zhang, C. Mao, S. Liao, Angew. Chem. Int. Ed. 2021, 60, 22035–22042; Angew. Chem. 2021, 133, 22206–22213.
- 9
- 9aH.-L. Qin, Q. Zheng, G. A. L. Bare, P. Wu, K. B. Sharpless, Angew. Chem. Int. Ed. 2016, 55, 14155–14158; Angew. Chem. 2016, 128, 14361–14364;
- 9bG.-F. Zha, Q. Zheng, J. Leng, P. Wu, H.-L. Qin, K. B. Sharpless, Angew. Chem. Int. Ed. 2017, 56, 4849–4852; Angew. Chem. 2017, 129, 4927–4930;
- 9cP. K. Chinthakindi, K. B. Govender, A. S. Kumar, H. G. Kruger, T. Govender, T. Naicker, P. I. Arvidsson, Org. Lett. 2017, 19, 480–483;
- 9dJ. Leng, H.-L. Qin, Chem. Commun. 2018, 54, 4477–4480;
- 9eC. J. Smedley, M.-C. Giel, A. Molino, A. S. Barrow, D. J. D. Wilson, J. E. Moses, Chem. Commun. 2018, 54, 6020–6023;
- 9fJ. Thomas, V. V. Fokin, Org. Lett. 2018, 20, 3749–3752;
- 9gB. Moku, W.-Y. Fang, J. Leng, E. A. B. Kantchev, H.-L. Qin, ACS Catal. 2019, 9, 10477–10488;
- 9hJ. Chen, B.-Q. Huang, Z.-Q. Wang, X.-J. Zhang, M. Yan, Org. Lett. 2019, 21, 9742–9746;
- 9iX.-Y. Chen, Y. Wu, J. Zhou, P. Wang, J.-Q. Yu, Org. Lett. 2019, 21, 1426–1429;
- 9jR. Xu, T. Xu, M. Yang, T. Cao, S. Liao, Nat. Commun. 2019, 10, 3752;
- 9kC. J. Smedley, G. Li, A. S. Barrow, T. L. Gialelis, M. C. Giel, A. Ottonello, Y. Cheng, S. Kitamura, D. W. Wolan, K. B. Sharpless, J. E. Moses, Angew. Chem. Int. Ed. 2020, 59, 12460–12469; Angew. Chem. 2020, 132, 12560–12569.
- 10
- 10aT. Govender, P. I. Arvidsson, G. E. M. Maguire, H. G. Kruger, T. Naicker, Chem. Rev. 2016, 116, 9375–9437;
- 10bD. Bonne, Y. Coquerel, T. Constantieux, J. Rodriguez, Tetrahedron: Asymmetry 2010, 21, 1085–1109;
- 10cB. I. Roman, N. D. Kimpe, C. V. Stevens, Chem. Rev. 2010, 110, 5914–5988.
- 11T. Henkel, T. Krügerke, K. Seppelt, Angew. Chem. Int. Ed. Engl. 1990, 29, 1128–1129; Angew. Chem. 1990, 102, 1171–1172.
- 12X. Zeng, H. Beckers, H. Willner, J. Am. Chem. Soc. 2013, 135, 2096–2099.
- 13
- 13aL. F. T. Novaes, J. Liu, Y. Shen, L. Lu, J. M. Meinhardt, S. Lin, Chem. Soc. Rev. 2021, 50, 7941–8002;
- 13bJ. Liu, L. Lu, D. Wood, S. Lin, ACS Cent. Sci. 2020, 6, 1317–1340;
- 13cD. Pollok, S. R. Waldvogel, Chem. Sci. 2020, 11, 12386–12400;
- 13dR. D. Little, J. Org. Chem. 2020, 85, 13375–13390;
- 13eC. Kingston, M. D. Palkowitz, Y. Takahira, J. C. Vantourout, B. K. Peters, Y. Kawamata, P. S. Baran, Acc. Chem. Res. 2020, 53, 72–83;
- 13fA. Wiebe, T. Gieshoff, S. Möhle, E. Rodrigo, M. Zirbes, S. R. Waldvogel, Angew. Chem. Int. Ed. 2018, 57, 5594–5619; Angew. Chem. 2018, 130, 5694–5721;
- 13gM. Yan, Y. Kawamata, P. S. Baran, Chem. Rev. 2017, 117, 13230–13319;
- 13hR. Francke, R. D. Little, Chem. Soc. Rev. 2014, 43, 2492–2521.
- 14
- 14aZ.-W. Hou, H.-C. Xu, Chin. J. Chem. 2020, 38, 394–398;
- 14bF. Xu, H. Long, J. Song, H.-C. Xu, Angew. Chem. Int. Ed. 2019, 58, 9017–9021; Angew. Chem. 2019, 131, 9115–9119;
- 14cH. Mei, Z. Yin, J. Liu, H. Sun, J. Han, Chin. J. Chem. 2019, 37, 292–301;
- 14dY. Yuan, A. Yao, Y. Zheng, M. Gao, Z. Zhou, J. Qiao, J. Hu, B. Ye, J. Zhao, H. Wen, A. Lei, iScience 2019, 12, 293–303;
- 14eW. Jud, C. O. Kappe, D. Cantillo, Org. Biomol. Chem. 2019, 17, 3529–3537;
- 14fF. Xu, Y.-J. Li, C. Huang, H.-C. Xu, ACS Catal. 2018, 8, 3820–3824;
- 14gP. Xiong, H.-H. Xu, J. Song, H.-C. Xu, J. Am. Chem. Soc. 2018, 140, 2460–2464;
- 14hZ.-W. Hou, Z.-Y. Mao, Y. Y. Melcamu, X. Lu, H.-C. Xu, Angew. Chem. Int. Ed. 2018, 57, 1636–1639; Angew. Chem. 2018, 130, 1652–1655;
- 14iJ. Wen, W. Shi, F. Zhang, D. Liu, S. Tang, H. Wang, X.-M. Lin, A. Lei, Org. Lett. 2017, 19, 3131–3134;
- 14jZ.-W. Hou, Z.-Y. Mao, H.-B. Zhao, Y. Y. Melcamu, X. Lu, J. Song, H.-C. Xu, Angew. Chem. Int. Ed. 2016, 55, 9168–9172; Angew. Chem. 2016, 128, 9314–9318.
- 15
- 15aX. Liu, C. Liu, X. Cheng, Green Chem. 2021, 23, 3468–3473;
- 15bL. Chang, J. Li, N. Wu, X. Cheng, Org. Biomol. Chem. 2021, 19, 2468–2472;
- 15cW. Zhang, S. Lin, J. Am. Chem. Soc. 2020, 142, 20661–20670;
- 15dL. Lu, J. C. Siu, Y. Lai, S. Lin, J. Am. Chem. Soc. 2020, 142, 21272–21278;
- 15eH. Kim, H. Kim, T. H. Lambert, S. Lin, J. Am. Chem. Soc. 2020, 142, 2087–2092;
- 15fX. Liu, R. Liu, J. Qiu, X. Cheng, G. Li, Angew. Chem. Int. Ed. 2020, 59, 13962–13967; Angew. Chem. 2020, 132, 14066–14071;
- 15gW. Liu, W. You, Y. Gong, Y. Deng, Energy Environ. Sci. 2020, 13, 917–927;
- 15hD. Lehnherr, Y.-H. Lam, M. C. Nicastri, J. Liu, J. A. Newman, E. L. Regalado, D. A. DiRocco, T. Rovis, J. Am. Chem. Soc. 2020, 142, 468–478;
- 15iK.-J. Jiao, D. Liu, H.-X. Ma, H. Qiu, P. Fang, T.-S. Mei, Angew. Chem. Int. Ed. 2020, 59, 6520–6524; Angew. Chem. 2020, 132, 6582–6586;
- 15jX.-T. Gao, Z. Zhang, X. Wang, J.-S. Tian, S.-L. Xie, F. Zhou, J. Zhou, Chem. Sci. 2020, 11, 10414–10420;
- 15kN. G. W. Cowper, C. P. Chernowsky, O. P. Williams, Z. K. Wickens, J. Am. Chem. Soc. 2020, 142, 2093–2099;
- 15lX. Chong, C. Liu, Y. Huang, C. Huang, B. Zhang, Nat. Sci. Rev. 2020, 7, 285–295;
- 15mJ. Li, L. He, X. Liu, X. Cheng, G. Li, Angew. Chem. Int. Ed. 2019, 58, 1759–1763; Angew. Chem. 2019, 131, 1773–1777;
- 15nE. Rodrigo, S. R. Waldvogel, Chem. Sci. 2019, 10, 2044–2047;
- 15oB. K. Peters, K. X. Rodriguez, S. H. Reisberg, S. B. Beil, D. P. Hickey, Y. Kawamata, M. Collins, J. Starr, L. Chen, S. Udyavara, K. Klunder, T. J. Gorey, S. L. Anderson, M. Neurock, S. D. Minteer, P. S. Baran, Science 2019, 363, 838–845;
- 15pB. Li, H. Ge, Sci. Adv. 2019, 5, eaaw2774.
- 16D. M. Heard, A. J. J. Lennox, Angew. Chem. Int. Ed. 2020, 59, 18866–18884; Angew. Chem. 2020, 132, 19026–19044.
- 17
- 17aX. Meng, Y. Zhang, J. Luo, F. Wang, X. Cao, S. Huang, Org. Lett. 2020, 22, 1169–1174;
- 17bD. Wang, Z. Wan, H. Zhang, A. Lei, Adv. Synth. Catal. 2021, 363, 1022–1027.
- 18M. Yu, H. Wang, Y. Gao, F. Bu, H. Cong, A. Lei, Cell Rep. Phys. Sci. 2021, 2, 100476.
- 19Deposition Numbers 2095408 (for 3l), 2095414 (for 4j), and 2095415 (for 10) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
- 20
- 20aD. E. Fahrney, A. M. Gold, J. Am. Chem. Soc. 2002, 124, 997–1000;
- 20bJ. C. Powers, J. L. Asgian, Ö. D. Ekici, K. E. James, Chem. Rev. 2002, 102, 4639–4750.
- 21
- 21aR. K. Boeckman, P. Ge, J. E. Reed, Org. Lett. 2001, 3, 3647–3650;
- 21bR. K. Boeckman, J. E. Reed, P. Ge, Org. Lett. 2001, 3, 3651–3653.
- 22
- 22aB. Swain, A. Angeli, P. Singh, C. T. Supuran, M. Arifuddin, Bioorg. Med. Chem. 2020, 28, 115586;
- 22bM. Krasavin, R. Zalubovskis, A. Grandane, I. Domraceva, P. Zhmurov, C. T. Supuran, J. Enzyme Inhib. Med. Chem. 2020, 35, 506–510;
- 22cK. Tars, D. Vullo, A. Kazaks, J. Leitans, A. Lends, A. Grandane, R. Zalubovskis, A. Scozzafava, C. T. Supuran, J. Med. Chem. 2013, 56, 293–300;
- 22dM. Tanc, F. Carta, M. Bozdag, A. Scozzafava, C. T. Supuran, Bioorg. Med. Chem. 2013, 21, 4502–4510.
- 23
- 23aQ. Lu, J. Zhang, G. Zhao, Y. Qi, H. Wang, A. Lei, J. Am. Chem. Soc. 2013, 135, 11481–11484;
- 23bX. Gong, C. Zhu, L.-W. Ye, Org. Biomol. Chem. 2020, 18, 1843–1850;
- 23cS. Handa, J. C. Fennewald, B. H. Lipshutz, Angew. Chem. Int. Ed. 2014, 53, 3432–3435; Angew. Chem. 2014, 126, 3500–3503.
- 24
- 24aC. Wang, F. Cao, Y. Ruan, X. Jia, W. Zhen, X. Jiang, Angew. Chem. Int. Ed. 2019, 58, 9846–9850; Angew. Chem. 2019, 131, 9951–9955;
- 24bG. A. Russell, J. Am. Chem. Soc. 2002, 124, 3871–3877.
- 25
- 25aB.-N. Kim, J. Kim, J.-Y. Ahn, S. Kim, B.-K. Cho, Y.-H. Kim, J. Min, Toxicol. Environ. Health Sci. 2020, 12, 297–304;
- 25bJ.-P. Lee, S. S. Sekhon, J. H. Kim, S. C. Kim, B.-K. Cho, J.-Y. Ahn, Y.-H. Kim, Mol. Cell. Toxicol. 2021, 17, 1–13.
- 26
- 26aP. F. Cannon, U. Damm, P. R. Johnston, B. S. Weir, Stud. Mycol. 2012, 73, 181–213;
- 26bB. S. Weir, P. R. Johnston, U. Damm, Stud. Mycol. 2012, 73, 115–180.