Three-Component Reaction to 1,4,2-Diazaborole-Type Heteroarene Systems
Dr. Jun Li
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstraβe 40, 48149 Münster, Germany
Search for more papers by this authorDr. Constantin G. Daniliuc
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstraβe 40, 48149 Münster, Germany
Search for more papers by this authorDr. Gerald Kehr
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstraβe 40, 48149 Münster, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Gerhard Erker
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstraβe 40, 48149 Münster, Germany
Search for more papers by this authorDr. Jun Li
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstraβe 40, 48149 Münster, Germany
Search for more papers by this authorDr. Constantin G. Daniliuc
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstraβe 40, 48149 Münster, Germany
Search for more papers by this authorDr. Gerald Kehr
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstraβe 40, 48149 Münster, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Gerhard Erker
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstraβe 40, 48149 Münster, Germany
Search for more papers by this authorGraphical Abstract
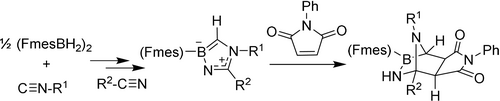
The reaction product between the bulky FmesBH2 borane and an isonitrile serves as a B/N frustrated Lewis pair that adds nitriles to form the dihydro-1,4,2-diazaborole heteroarene derivatives. One example of this class of compounds was shown to undergo a cycloaddition reaction with N-phenylmaleimide.
Abstract
The borane FmesBH2 reacts in a three-component reaction with an isonitrile and a small series of organonitriles to give rare examples of the class of dihydro-1,4,2-diazaborole derivatives. In a related way, annulated BN-indolizine derivatives became conveniently available, as were dihydro-1,4,2-oxaza- or thiazaborole derivatives. The nucleophilic framework of a dihydro-1,4,2-diazaborole example allowed for an uncatalyzed acylation reaction. It also served as a 1,3-dipolar reagent and underwent a [3+2] cycloaddition/[4+2] cycloreversion sequence when treated with methyl propiolate to give the respective pyrrole product. The [3+2] cycloaddition product of a dihydro-1,4,2-diazaborole derivative with N-phenylmaleimide was isolated and its heterobicyclo[2.2.1]heptane derived structure characterized by X-ray diffraction.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202111946-sup-0001-misc_information.pdf5.8 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. J. D. Bosdet, W. E. Piers, Can. J. Chem. 2009, 87, 8–29;
- 1bP. G. A. Campbell, J. V. Marwitz, S.-Y. Liu, Angew. Chem. Int. Ed. 2012, 51, 6074–6092; Angew. Chem. 2012, 124, 6178–6197;
- 1cG. Bélanger-Chabot, H. Braunschweig, D. K. Roy, Eur. J. Inorg. Chem. 2017, 4353–4368;
- 1dB. Su, R. Kinjo, Synthesis 2017, 49, 2985–3034;
- 1eZ. X. Giustra, S.-Y. Liu, J. Am. Chem. Soc. 2018, 140, 1184–1194.
- 2
- 2aB. Su, Y. Li, R. Ganguly, J. Lim, R. Kinjo, J. Am. Chem. Soc. 2015, 137, 11274–11277;
- 2bB. Su, Y. Li, R. Ganguly, R. Kinjo, Angew. Chem. Int. Ed. 2017, 56, 14572–14576; Angew. Chem. 2017, 129, 14764–14768.
- 3
- 3aG. Hesse, H. Witte, W. Gulden, Tetrahedron Lett. 1966, 7, 2707–2710;
10.1016/S0040-4039(00)62011-7 Google Scholar
- 3bY. Yamamoto, K. Kondo, I. Moritani, Bull. Chem. Soc. Jpn. 1975, 48, 3682–3685;
- 3cU. Sicker, A. Meller, W. Maringgele, J. Organomet. Chem. 1982, 231, 191–203;
- 3dM. Suginome, T. Fukuda, H. Nakamura, Y. Ito, Organometallics 2000, 19, 719–721;
- 3eM. Suginome, T. Fukuda, Y. Ito, J. Organomet. Chem. 2002, 643–644, 508–511.
- 4B. R. Barnett, C. E. Moore, A. L. Rheingold, J. S. Figueroa, Chem. Commun. 2015, 51, 541–544.
- 5B. R. Barnett, C. E. Moore, A. L. Rheingold, J. S. Figueroa, J. Am. Chem. Soc. 2014, 136, 10262–10265.
- 6J. Li, C. G. Daniliuc, G. Kehr, G. Erker, Angew. Chem. Int. Ed. 2019, 58, 6737–6741; Angew. Chem. 2019, 131, 6809–6813.
- 7
- 7aJ. Li, C. G. Daniliuc, C. Mück-Lichtenfeld, G. Kehr, G. Erker, Angew. Chem. Int. Ed. 2019, 58, 15377–15380; Angew. Chem. 2019, 131, 15521–15524;
- 7bJ. Li, C. G. Daniliuc, K. K. Kartha, G. Fernández, G. Kehr, G. Erker, J. Am. Chem. Soc. 2021, 143, 2059–2067.
- 8
- 8aJ. Li, C. Mück-Lichtenfeld, C. G. Daniliuc, G. Kehr, G. Erker, Angew. Chem. Int. Ed. 2020, 59, 12477–12483; Angew. Chem. 2020, 132, 12577–12583;
- 8bQ. Sun, C. G. Daniliuc, C. Mück-Lichtenfeld, K. Bergander, G. Kehr, G. Erker, J. Am. Chem. Soc. 2020, 142, 17260–17264;
- 8cK. Škoch, C. G. Daniliuc, G. Kehr, G. Erker, Angew. Chem. Int. Ed. 2021, 60, 6757–6763; Angew. Chem. 2021, 133, 6831–6837.
- 9
- 9aD. W. Stephan, G. Erker, Angew. Chem. Int. Ed. 2015, 54, 6400–6441; Angew. Chem. 2015, 127, 6498–6541;
- 9bD. W. Stephan, Acc. Chem. Res. 2015, 48, 306–316;
- 9cD. W. Stephan, Science 2016, 354, aaf7229;
- 9d Frustrated Lewis Pairs. Molecular Catalysis, Vol. 2. (Eds.: J. C. Slootweg, A. R. Jupp), Springer, Cham, Switzerland, 2020.
- 10
- 10aA. Stute, G. Kehr, R. Fröhlich, G. Erker, Chem. Commun. 2011, 47, 4288–4290;
- 10bC. Appelt, H. Westenberg, F. Bertini, A. W. Ehlers, J. C. Slootweg, K. Lammertsma, W. Uhl, Angew. Chem. Int. Ed. 2011, 50, 3925–3928; Angew. Chem. 2011, 123, 4011–4014;
- 10cF. Bertini, V. B. Lyaskovskyy, J. J. Timmer, F. J. J. de Kanter, M. Lutz, A. W. Ehlers, J. C. Slootweg, K. Lammertsma, J. Am. Chem. Soc. 2012, 134, 201–204;
- 10dX. Xu, G. Kehr, C. G. Daniliuc, G. Erker, J. Am. Chem. Soc. 2013, 135, 6465–6476;
- 10eL. Fan, A. R. Jupp, D. W. Stephan, J. Am. Chem. Soc. 2018, 140, 8119–8123.
- 11
- 11aM. A. Dureen, D. W. Stephan, J. Am. Chem. Soc. 2010, 132, 13559–13568;
- 11bA. Hamza, K. Sorochkina, B. Kotai, K. Chernichenko, D. Berta, M. Bolte, M. Nieger, T. Repo, I. Pápai, ACS Catal. 2020, 10, 14290–14301.
- 12
- 12aM. A. Dureen, D. W. Stephan, J. Am. Chem. Soc. 2009, 131, 8396–8397;
- 12bC. Jiang, O. Blacque, H. Berke, Organometallics 2010, 29, 125–133;
- 12cK. Chernichenko, A. Madarász, I. Pápai, M. Nieger, M. Leskelä, T. Repo, Nat. Chem. 2013, 5, 718–723.
- 13
- 13aG. Hesse, H. Witte, Angew. Chem. Int. Ed. Engl. 1963, 2, 617–617;
10.1002/anie.196306172 Google ScholarAngew. Chem. 1963, 75, 791–792;
- 13bJ. Tanaka, J. C. Carter, Tetrahedron Lett. 1965, 6, 329–332;
10.1016/S0040-4039(01)83882-X Google Scholar
- 13cJ. Casanova, Jr., H. R. Kiefer, D. Kuwada, A. H. Boulton, Tetrahedron Lett. 1965, 6, 703–714;
10.1016/S0040-4039(01)83969-1 Google Scholar
- 13dK. Škoch, C. G. Daniliuc, G. Kehr, G. Erker, Chem. Commun. 2020, 56, 12178–12181.
- 14
- 14aL. Keweloh, H. Klöcker, E.-U. Würthwein, W. Uhl, Angew. Chem. Int. Ed. 2016, 55, 3212–3215; Angew. Chem. 2016, 128, 3266–3269;
- 14bJ. Möricke, B. Wibbeling, C. G. Daniliuc, G. Kehr, G. Erker, Philos. Trans. R. Soc. A 2017, 375, 20170015.
- 15NH⋅⋅⋅N type hydrogen bonds widely distribute in base pairs of nucleic acids with NN separation ranging from 2.8 Å to 3.0 Å, for example: T. Kobuna, T. Sunami, J. Kondo, A. Takénaka, Nucleic Acids Res. Suppl. 2002, 2, 179–180.
- 16
- 16aE. R. Abbey, L. N. Zakharov, S.-Y. Liu, J. Am. Chem. Soc. 2010, 132, 16340–16342;
- 16bE. R. Abbey, L. N. Zakharov, S.-Y. Liu, J. Am. Chem. Soc. 2011, 133, 11508–11511;
- 16cS. M. McDonald, S. K. Mellerup, J. Peng, D. Yang, Q.-S. Li, S. Wang, Chem. Eur. J. 2015, 21, 13961–13970.
- 17For review, see: S. Rohrbach, A. J. Smith, J. H. Pang, D. L. Poole, T. Tuttle, S. Chiba, J. A. Murphy, Angew. Chem. Int. Ed. 2019, 58, 16368–16388; Angew. Chem. 2019, 131, 16518–16540, and references therein.
- 18Examples:
- 18aK. Samigullin, I. Georg, M. Bolte, H.-W. Lerner, M. Wagner, Chem. Eur. J. 2016, 22, 3478–3484;
- 18bM. Devillard, R. Declercq, E. Nicolas, A. W. Ehlers, J. Backs, N. Saffon-Merceron, G. Bouhadir, J. C. Slootweg, W. Uhl, D. Bourissou, J. Am. Chem. Soc. 2016, 138, 4917–4926;
- 18cP. Holtkamp, F. Friedrich, E. Stratmann, A. Mix, B. Neumann, H.-G. Stammler, N. W. Mitzel, Angew. Chem. Int. Ed. 2019, 58, 5114–5118; Angew. Chem. 2019, 131, 5168–5172.
- 19Dihydro-1,4,2-thiazaborole 16 seems to represent an example of a previously unknown class of heterocyclic compounds. See for a comparison: C. D. Habben, Z. Anorg. Allg. Chem. 1989, 573, 199–207.
- 20The B=C bond in those compounds formally correspond to borataalkene subunits and, consequently, show a related reactivity. See e.g.:
- 20aR. A. Bartlett, P. P. Power, Organometallics 1986, 5, 1916–1917;
- 20bM. M. Olmstead, P. P. Power, K. J. Weese, R. J. Doedens, J. Am. Chem. Soc. 1987, 109, 2541–2542;
- 20cM. Pilz, J. Allwohn, R. Hunold, W. Massa, A. Berndt, Angew. Chem. Int. Ed. Engl. 1988, 27, 1370–1372; Angew. Chem. 1988, 100, 1421–1422;
- 20dC.-W. Chiu, F. P. Gabbaï, Angew. Chem. Int. Ed. 2007, 46, 6878–6881; Angew. Chem. 2007, 119, 7002–7005;
- 20eP. Moquist, G.-Q. Chen, C. Mück-Lichtenfeld, K. Bussmann, C. G. Daniliuc, G. Kehr, G. Erker, Chem. Sci. 2015, 6, 816–825;
- 20fS. Kohrt, S. Dachwitz, C. G. Daniliuc, G. Kehr, G. Erker, Dalton Trans. 2015, 44, 21032–21040;
- 20gT. Wang, S. Kohrt, C. G. Daniliuc, G. Kehr, G. Erker, Org. Biomol. Chem. 2017, 15, 6223–6232.
- 21M. B. Teimouri, F. Zolfaghari, S. Naderi, Tetrahedron 2017, 73, 262–271.
- 22
- 22aR. Huisgen, Angew. Chem. Int. Ed. Engl. 1963, 2, 565–598;
10.1002/anie.196305651 Google ScholarAngew. Chem. 1963, 75, 604–637;
- 22bR. Huisgen, Angew. Chem. Int. Ed. Engl. 1963, 2, 633–645;
10.1002/anie.196306331 Google ScholarAngew. Chem. 1963, 75, 742–754.
- 23B. Merah, F. Texier, Bull. Soc. Chim. Fr. Part 2 1980, 11–12, 552–558.
- 24
- 24aK. Alder, H. F. Rickert, Ber. Dtsch. Chem. Ges. 1937, 70, 1354–1363;
- 24bB. Rickborn, Org. React. 1998, 52, 1–393;
- 24cB. Rickborn, Org. React. 1998, 53, 223–629;
- 24dM. Breugst, H.-U. Reißig, Angew. Chem. Int. Ed. 2020 , 59, 12389–12404; Angew. Chem. 2020, 132, 12389–12404.
- 25
- 25aF. A. Tsao, L. Cao, S. Grimme, D. W. Stephan, J. Am. Chem. Soc. 2015, 137, 13264–13267;
- 25bS. Dong, C. G. Daniliuc, G. Kehr, G. Erker, Chem. Eur. J. 2020, 26, 745–753.
- 26
- 26aP. Paetzold, C. Von Plotho, G. Schmid, R. Boese, B. Schrader, D. Bougeard, U. Pfeiffer, R. Gleiter, W. Schüfer, Chem. Ber. 1984, 117, 1089–1102;
- 26bM. Haase, U. Klingebiel, Angew. Chem. Int. Ed. Engl. 1985, 24, 324; Angew. Chem. 1985, 97, 335.
- 27
- 27aK. Alder, G. Stein, Angew. Chem. 1937, 50, 510–519;
- 27bK. N. Houk, Tetrahedron Lett. 1970, 11, 2621–2624.
10.1016/S0040-4039(01)98296-6 Google Scholar
- 28
- 28aA. Dömling, I. Ugi, Angew. Chem. Int. Ed. 2000, 39, 3168–3210;
10.1002/1521-3773(20000915)39:18<3168::AID-ANIE3168>3.0.CO;2-U CAS PubMed Web of Science® Google ScholarAngew. Chem. 2000, 112, 3300–3344;
- 28bQ. Wang, D.-X. Wang, M.-X. Wang, J. Zhu, Acc. Chem. Res. 2018, 51, 1290–1300, and references therein;
- 28cN. Kielland, F. Catti, D. Bello, N. Isambert, I. Soteras, F. J. Luque, R. Lavilla, Chem. Eur. J. 2010, 16, 7904–7915.
- 29
- 29aG. M. Clark, K. G. Hancock, G. Zweifel, J. Am. Chem. Soc. 1971, 93, 1308–1309;
- 29bA. Iida, S. Saito, T. Sasamori, S. Yamaguchi, Angew. Chem. Int. Ed. 2013, 52, 3760–3764; Angew. Chem. 2013, 125, 3848–3852;
- 29cJ. Möbus, G. Kehr, C. G. Daniliuc, C. Mück-Lichtenfeld, G. Erker, Angew. Chem. Int. Ed. 2015, 54, 12366–12369; Angew. Chem. 2015, 127, 12543–12546;
- 29dF. Ge, F. Türkyilmaz, C. G. Daniliuc, M. Siedow, H. Eckert, G. Kehr, G. Erker, Chem. Asian J. 2015, 10, 2497–2502.
- 30
- 30aH. Jacobsen, H. Berke, S. Doering, G. Kehr, G. Erker, R. Fröhlich, O. Meyer, Organometallics 1999, 18, 1724–1735;
- 30bO. Ekkert, G. G. Miera, T. Wiegand, H. Eckert, B. Schirmer, J. L. Petersen, C. G. Daniliuc, R. Fröhlich, S. Grimme, G. Kehr, G. Erker, Chem. Sci. 2013, 4, 2657–2664.
- 31
- 31aS. Dong, L. Wang, T. Wang, C. G. Daniliuc, M. Brinkkötter, H. Eckert, G. Kehr, G. Erker, Dalton Trans. 2018, 47, 4449–4454;
- 31bX. Jie, C. G. Daniliuc, R. Knitsch, M. R. Hansen, H. Eckert, S. Ehlert, S. Grimme, G. Kehr, G. Erker, Angew. Chem. Int. Ed. 2019, 58, 882–886; Angew. Chem. 2019, 131, 892–896.
- 32CCDC deposition numbers 2077484, 2077485, 2077486, 2077487, 2077488, 2077489, 2077490, 2077491, 2077492, 2077493, 2077494, 2077495, 2077496, 2077497, 2077498, 2077499, and 2077500 contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.