Cobalt-Catalysed Asymmetric Addition and Alkylation of Secondary Phosphine Oxides for the Synthesis of P-Stereogenic Compounds
Zeng-Hua Wu
School of Chemistry and Chemical Engineering, Shaanxi Normal University, 620 Xi Changan Street, Xi'an, 710119 China
Search for more papers by this authorAn-Qi Cheng
School of Chemistry and Chemical Engineering, Shaanxi Normal University, 620 Xi Changan Street, Xi'an, 710119 China
Search for more papers by this authorMeng Yuan
School of Chemistry and Chemical Engineering, Shaanxi Normal University, 620 Xi Changan Street, Xi'an, 710119 China
Search for more papers by this authorYa-Xuan Zhao
School of Chemistry and Chemical Engineering, Shaanxi Normal University, 620 Xi Changan Street, Xi'an, 710119 China
Search for more papers by this authorHuai-Lan Yang
School of Chemistry and Chemical Engineering, Shaanxi Normal University, 620 Xi Changan Street, Xi'an, 710119 China
Search for more papers by this authorLi-Hua Wei
School of Chemistry and Chemical Engineering, Shaanxi Normal University, 620 Xi Changan Street, Xi'an, 710119 China
Search for more papers by this authorHuai-Yu Wang
School of Chemistry and Chemical Engineering, Shaanxi Normal University, 620 Xi Changan Street, Xi'an, 710119 China
Search for more papers by this authorDr. Tao Wang
School of Chemistry and Chemical Engineering, Shaanxi Normal University, 620 Xi Changan Street, Xi'an, 710119 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Zunting Zhang
School of Chemistry and Chemical Engineering, Shaanxi Normal University, 620 Xi Changan Street, Xi'an, 710119 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Wei-Liang Duan
School of Chemistry and Chemical Engineering, Shaanxi Normal University, 620 Xi Changan Street, Xi'an, 710119 China
College of Chemistry and Chemical Engineering, Yangzhou University, 180 Siwangting Road, Yangzhou, 225002 China
Search for more papers by this authorZeng-Hua Wu
School of Chemistry and Chemical Engineering, Shaanxi Normal University, 620 Xi Changan Street, Xi'an, 710119 China
Search for more papers by this authorAn-Qi Cheng
School of Chemistry and Chemical Engineering, Shaanxi Normal University, 620 Xi Changan Street, Xi'an, 710119 China
Search for more papers by this authorMeng Yuan
School of Chemistry and Chemical Engineering, Shaanxi Normal University, 620 Xi Changan Street, Xi'an, 710119 China
Search for more papers by this authorYa-Xuan Zhao
School of Chemistry and Chemical Engineering, Shaanxi Normal University, 620 Xi Changan Street, Xi'an, 710119 China
Search for more papers by this authorHuai-Lan Yang
School of Chemistry and Chemical Engineering, Shaanxi Normal University, 620 Xi Changan Street, Xi'an, 710119 China
Search for more papers by this authorLi-Hua Wei
School of Chemistry and Chemical Engineering, Shaanxi Normal University, 620 Xi Changan Street, Xi'an, 710119 China
Search for more papers by this authorHuai-Yu Wang
School of Chemistry and Chemical Engineering, Shaanxi Normal University, 620 Xi Changan Street, Xi'an, 710119 China
Search for more papers by this authorDr. Tao Wang
School of Chemistry and Chemical Engineering, Shaanxi Normal University, 620 Xi Changan Street, Xi'an, 710119 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Zunting Zhang
School of Chemistry and Chemical Engineering, Shaanxi Normal University, 620 Xi Changan Street, Xi'an, 710119 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Wei-Liang Duan
School of Chemistry and Chemical Engineering, Shaanxi Normal University, 620 Xi Changan Street, Xi'an, 710119 China
College of Chemistry and Chemical Engineering, Yangzhou University, 180 Siwangting Road, Yangzhou, 225002 China
Search for more papers by this authorGraphical Abstract
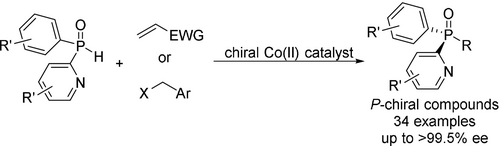
The catalytic asymmetric synthesis of P-chiral phosphorus compounds is an important way to construct P-chiral ligands. Herein, we report a new strategy that adopts the pyridinyl moiety as the coordinating group in the cobalt-catalysed asymmetric nucleophilic addition/alkylation of secondary phosphine oxides (SPOs). A series of tertiary phosphine oxides (TPOs) were generated with up to 99 % yield and 99.5 % ee, and with broad functional-group tolerance.
Abstract
The catalytic asymmetric synthesis of P-chiral phosphorus compounds is an important way to construct P-chiral ligands. Herein, we report a new strategy that adopts the pyridinyl moiety as the coordinating group in the cobalt-catalysed asymmetric nucleophilic addition/alkylation of secondary phosphine oxides. A series of tertiary phosphine oxides were generated with up to 99 % yield and 99.5 % ee, and with broad functional-group tolerance. Mechanistic studies reveal that (R)-secondary phosphine oxides preferentially interact with the cobalt catalysts to produce P-stereogenic compounds.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202111137-sup-0001-misc_information.pdf12.7 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aW. Tang, X. Zhang, Chem. Rev. 2003, 103, 3029–3070;
- 1b Phosphorus Ligands in Asymmetric Catalysis: Synthesis and Applications, Vols. 1–3 (Eds.: A. Börner), Wiley-VCH, Weinheim, 2008;
- 1cH. Fernández-Pérez, P. Etayo, A. Panossian, A. Vidal-Ferran, Chem. Rev. 2011, 111, 2119–2176;
- 1d Privileged Chiral Ligands and Catalysts, Vol. 6 (Eds.: Q.-L. Zhou), Wiley-VCH, Weinheim, 2011;
10.1002/9783527635207 Google Scholar
- 1e“The Use of New Phosphines as Powerful Tools in Asymmetric Synthesis of Biologically Active Compounds”: C. A. Busacca, C. H. Senanayake, Comprehensive Chirality, Vol. 1 (Eds.: E. M. Carreira, H. Yamamoto), Elsevier, Amsterdam, 2012, pp. 167–216;
10.1016/B978-0-08-095167-6.00110-5 Google Scholar
- 1f Phosphorus(III) Ligands in Homogeneous Catalysis: Design and Synthesis (Eds.: P. C. J. Kamer, P. W. N. M. van Leeuwen), Wiley, Chichester, 2012;
10.1002/9781118299715 Google Scholar
- 1gM. Dutartre, J. Bayardon, S. Juge, Chem. Soc. Rev. 2016, 45, 5771–5794.
- 2
- 2aJ. L. Methot, W. R. Roush, Adv. Synth. Catal. 2004, 346, 1035–1050;
- 2bJ. Seayad, B. List, Org. Biomol. Chem. 2005, 3, 719–724;
- 2cS. J. Connon, Angew. Chem. Int. Ed. 2006, 45, 3909–3912; Angew. Chem. 2006, 118, 4013–4016;
- 2dE. Remond, S. Juge, Chim. Oggi 2014, 32, 49–55;
- 2eH. Guo, Y. C. Fan, Z. Sun, Y. Wu, O. Kwon, Chem. Rev. 2018, 118, 10049–10293;
- 2fH. Ni, W.-L. Chan, Y. Lu, Chem. Rev. 2018, 118, 9344–9411;
- 2gT. Ayad, A. Gernet, J.-L. Pirat, D. Virieux, Tetrahedron 2019, 75, 4385–4418.
- 3For recent reviews, see:
- 3aK. M. Pietrusiewicz, M. Zablocka, Chem. Rev. 1994, 94, 1375–1411;
- 3bA. Grabulosa, J. Granell, G. Muller, Coord. Chem. Rev. 2007, 251, 25–90;
- 3cO. I. Kolodiazhnyi, Tetrahedron: Asymmetry 2012, 23, 1–46; For recent examples, see:
- 3dE. Bergin, C. T. O'Connor, S. B. Robinson, E. M. McGarrigle, C. P. O'Mahony, D. G. Gilheany, J. Am. Chem. Soc. 2007, 129, 9566–9567;
- 3eZ.-S. Han, N. Goyal, M. A. Herbage, J. D. Sieber, B. Qu, Y. Xu, Z. Li, J. T. Reeves, J.-N. Desrosiers, S. Ma, N. Grinberg, H. Lee, H. P. R. Mangunuru, Y. Zhang, D. Krishnamurthy, B. Z. Lu, J. Song, G. Wang, C. H. Senanayake, J. Am. Chem. Soc. 2013, 135, 2474–2477;
- 3fS. Rast, B. Mohar, M. Stephan, Org. Lett. 2014, 16, 2688–2691;
- 3gZ. S. Han, H. Wu, Y. Xu, Y. Zhang, B. Qu, Z. Li, D. R. Caldwell, K. R. Fandrick, L. Zhang, F. Roschangar, J. Song, C. H. Senanayake, Org. Lett. 2017, 19, 1796–1799.
- 4For reviews on the synthesis of P-chiral phosphines, see:
- 4aD. S. Glueck, Chem. Eur. J. 2008, 14, 7108–7117;
- 4bJ. S. Harvey, V. Gouverneur, Chem. Commun. 2010, 46, 7477–7485;
- 4cD. Zhao, R. Wang, Chem. Soc. Rev. 2012, 41, 2095–2108;
- 4dZ. Li, W.-L. Duan, Chin. J. Org. Chem. 2016, 36, 1805–1813;
- 4eR.-Y. Zhu, K. Liao, J.-S. Yu, J. Zhou, Huaxue Xuebao 2020, 78, 193–216;
- 4fD. S. Glueck, Synlett 2021, 32, 875–884;
- 4gD. S. Glueck, Synthesis 2021, https://doi.org/10.1055/a-1582-0169.
- 5
- 5aZ.-J. Du, J. Guan, G.-J. Wu, P. Xu, L.-X. Gao, F.-S. Han, J. Am. Chem. Soc. 2015, 137, 632–635;
- 5bZ.-Q. Lin, W.-Z. Wang, S.-B. Yan, W.-L. Duan, Angew. Chem. Int. Ed. 2015, 54, 6265–6269; Angew. Chem. 2015, 127, 6363–6367;
- 5cY. S. Jang, M. Dieckmann, N. Cramer, Angew. Chem. Int. Ed. 2017, 56, 15088–15092; Angew. Chem. 2017, 129, 15284–15288;
- 5dY. S. Jang, L. Wozniak, J. Pedroni, N. Cramer, Angew. Chem. Int. Ed. 2018, 57, 12901–12905; Angew. Chem. 2018, 130, 13083–13087;
- 5eZ. Li, Z.-Q. Lin, C.-G. Yan, W.-L. Duan, Organometallics 2019, 38, 3916–3920;
- 5fY. H. Chen, X.-L. Qin, F.-S. Han, Chem. Commun. 2017, 53, 5826–5829;
- 5gY. Sun, N. Cramer, Chem. Sci. 2018, 9, 2981–2985.
- 6For selected examples, see:
- 6aK. M. Lim, T. Hayashi, J. Am. Chem. Soc. 2017, 139, 8122–8125;
- 6bY. Zheng, L. Guo, W. Zi, Org. Lett. 2018, 20, 7039–7043;
- 6cY. Zhang, F. Zhang, L. Chen, J. Xu, X. Liu, X. Feng, ACS Catal. 2019, 9, 4834–4840.
- 7J. S. Harvey, S. J. Malcolmson, K. S. Dunne, S. J. Meek, A. L. Thompson, R. R. Schrock, A. H. Hoveyda, V. Gouverneur, Angew. Chem. Int. Ed. 2009, 48, 762–766; Angew. Chem. 2009, 121, 776–780.
- 8G. Nishida, K. Noguchi, M. Hirano, K. Tanaka, Angew. Chem. Int. Ed. 2008, 47, 3410–3413; Angew. Chem. 2008, 120, 3458–3461.
- 9
- 9aY. Huang, Y. Li, P.-H. Leung, T. Hayashi, J. Am. Chem. Soc. 2014, 136, 4865–4868;
- 9bC. Li, Q.-L. Bian, S. Xu, W.-L. Duan, Org. Chem. Front. 2014, 1, 541–545;
- 9cW.-J. Yue, J.-Z. Xiao, S. Zhang, L. Yin, Angew. Chem. Int. Ed. 2020, 59, 7057–7062; Angew. Chem. 2020, 132, 7123–7128;
- 9dY.-B. Li, H. Tian, L. Yin, J. Am. Chem. Soc. 2020, 142, 20098–20106;
- 9eC. Wang, K. Huang, J. Ye, W.-L. Duan, J. Am. Chem. Soc. 2021, 143, 5685–5690.
- 10
- 10aV. S. Chan, I. C. Stewart, R. G. Bergman, F. D. Toste, J. Am. Chem. Soc. 2006, 128, 2786–2787;
- 10bC. Scriban, D. S. Glueck, J. Am. Chem. Soc. 2006, 128, 2788–2789;
- 10cB.-J. Anderson, M. A. Guino-O, D. S. Glueck, J. A. Golen, A. G. DiPasquale, L. M. Liable-Sands, A. L. Rheingold, Org. Lett. 2008, 10, 4425–4428;
- 10dB.-J. Anderson, M. A. Guino-O, D. S. Glueck, A. G. Dipasquale, A. L. Rheingold, Organometallics 2008, 27, 4992–5001;
- 10eV. S. Chan, M. Chiu, R. G. Bergman, F. D. Toste, J. Am. Chem. Soc. 2009, 131, 6021–6032;
- 10fB. J. Anderson, S. C. Reynolds, M. A. Guino-o, Z. Xu, D. S. Glueck, ACS Catal. 2016, 6, 8106–8114;
- 10gS. Zhang, J.-Z. Xiao, Y.-B. Li, C.-Y. Shi, L. Yin, J. Am. Chem. Soc. 2021, 143, 9912–9921.
- 11
- 11aJ. R. Moncarz, N. F. Laritcheva, D. S. Glueck, J. Am. Chem. Soc. 2002, 124, 13356–13357;
- 11bV. S. Chan, R. G. Bergman, F. D. Toste, J. Am. Chem. Soc. 2007, 129, 15122–15123;
- 11cN. F. Blank, J. R. Moncarz, T. J. Brunker, C. Scriban, B. J. Anderson, O. Amir, D. S. Glueck, L. N. Zakharov, J. A. Golen, C. D. Incarvito, A. L. Rheingold, J. Am. Chem. Soc. 2007, 129, 6847–6858;
- 11dT. J. Brunker, B. J. Anderson, N. F. Blank, D. S. Glueck, A. L. Rheingold, Org. Lett. 2007, 9, 1109–1112.
- 12R. Beaud, R. J. Phipps, M. J. Gaunt, J. Am. Chem. Soc. 2016, 138, 13183–13186.
- 13Y. Zhang, H. He, Q. Wang, Q. Cai, Tetrahedron Lett. 2016, 57, 5308–5311.
- 14
- 14aQ. Dai, B. Li, M. Li, L. Zhang, J. Am. Chem. Soc. 2019, 141, 20556–20564;
- 14bQ. Dai, L. Liu, Y. Qian, W. Li, J. Zhang, Angew. Chem. Int. Ed. 2020, 59, 20645–20650; Angew. Chem. 2020, 132, 20826–20831;
- 14cH. Qiu, Q. Dai, J. He, W. Li, J. Zhang, Chem. Sci. 2020, 11, 9983–9988.
- 15X.-T. Liu, Y.-Q. Zhang, X.-Y. Han, S.-P. Sun, Q.-W. Zhang, J. Am. Chem. Soc. 2019, 141, 16584–16589.
- 16
- 16aK. Dong, X. Fang, S. Gülak, R. Franke, A. Spannenberg, H. Neumann, R. Jackstell, M. Beller, Nat. Commun. 2017, 8, 14117–14123;
- 16bK. Dong, R. Sang, R. Franke, A. Spannenberg, H. Neumann, R. Jackstell, M. Beller, Angew. Chem. Int. Ed. 2017, 56, 5267–5271; Angew. Chem. 2017, 129, 5351–5355;
- 16cK. Dong, R. Sang, J. Liu, R. Razzaq, R. Franke, R. Jackstell, M. Beller, Angew. Chem. Int. Ed. 2017, 56, 6203–6207; Angew. Chem. 2017, 129, 6299–6303;
- 16dL. Wang, H. Neumann, M. Beller, Angew. Chem. Int. Ed. 2018, 57, 6910–6914; Angew. Chem. 2018, 130, 7026–7030;
- 16eR. Sang, P. Kucmierczyk, K. Dong, R. Franke, H. Neumann, R. Jackstell, M. Beller, J. Am. Chem. Soc. 2018, 140, 5217–5223;
- 16fJ. Liu, K. Dong, R. Franke, H. Neumann, R. Jackstell, M. Beller, J. Am. Chem. Soc. 2018, 140, 10282–10288;
- 16gJ. Yang, J. Liu, H. Neumann, R. Franke, R. Jackstell, M. Beller, Science 2019, 366, 1514–1517;
- 16hJ. Liu, J. Yang, W. Baumann, R. Jackstell, M. Beller, Angew. Chem. Int. Ed. 2019, 58, 10683–10687; Angew. Chem. 2019, 131, 10793–10797;
- 16iR. Sang, P. Kucmierczyk, R. Dühren, R. Razzaq, K. Dong, J. Liu, R. Franke, R. Jackstell, M. Beller, Angew. Chem. Int. Ed. 2019, 58, 14365–14373; Angew. Chem. 2019, 131, 14503–14511;
- 16jJ.-W. Liu, C.-L. Schneider, J. Yang, Z.-H. Wei, H.-J. Jiao, R. Franke, R. Jackstell, M. Beller, Angew. Chem. Int. Ed. 2021, 60, 371–379; Angew. Chem. 2021, 133, 375–383;
- 16kJ.-W. Liu, J. Yang, C. Schneider, R. Franke, R. Jackstell, M. Beller, Angew. Chem. Int. Ed. 2020, 59, 9032–9040; Angew. Chem. 2020, 132, 9117–9125.
- 17
- 17aQ.-H. Deng, H. Wadepohl, L. H. Gade, Chem. Eur. J. 2011, 17, 14922–14928;
- 17bQ.-H. Deng, H. Wadepohl, L. H. Gade, J. Am. Chem. Soc. 2012, 134, 2946–2949;
- 17cQ.-H. Deng, H. Wadepohl, L. H. Gade, J. Am. Chem. Soc. 2012, 134, 10769–10772;
- 17dQ.-H. Deng, T. Bleith, H. Wadepohl, L. H. Gade, J. Am. Chem. Soc. 2013, 135, 5356–5359;
- 17eQ.-H. Deng, C. Rettenmeier, H. Wadepohl, L. H. Gade, Chem. Eur. J. 2014, 20, 93–97;
- 17fT. Bleith, H. Wadepohl, L. H. Gade, J. Am. Chem. Soc. 2015, 137, 2456–2459;
- 17gT. Bleith, L. H. Gade, J. Am. Chem. Soc. 2016, 138, 4972–4983;
- 17hT. Bleith, Q.-H. Deng, H. Wadepohl, L. H. Gade, Angew. Chem. Int. Ed. 2016, 55, 7852–7856; Angew. Chem. 2016, 128, 7983–7987;
- 17iV. Vasilenko, C. K. Blasius, H. Wadepohl, L. H. Gade, Angew. Chem. Int. Ed. 2017, 56, 8393–8397; Angew. Chem. 2017, 129, 8513–8517;
- 17jC. K. Blasius, V. Vasilenko, L.-H. Gade, Angew. Chem. Int. Ed. 2018, 57, 10231–10235; Angew. Chem. 2018, 130, 10388–10392;
- 17kC. K. Blasius, N. F. Heinrich, V. Vasilenko, L. H. Gade, Angew. Chem. Int. Ed. 2020, 59, 15974–15977; Angew. Chem. 2020, 132, 16108–16111.
- 18C. K. Blasius, H. Wadepohl, L. H. Gade, Eur. J. Inorg. Chem. 2020, 2335–2342.
- 19Deposition Number 2059262 (for 3bg) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
- 20A. Hu, H. Ngo, W. Lin, Angew. Chem. Int. Ed. 2004, 43, 2501–2504; Angew. Chem. 2004, 116, 2555–2558.
- 21Q. Xu, C.-Q. Zhao, L.-B. Han, J. Am. Chem. Soc. 2008, 130, 12648–12655.
- 22
- 22aE. Vedejs, M. Jure, Angew. Chem. Int. Ed. 2005, 44, 3974–4001; Angew. Chem. 2005, 117, 4040–4069;
- 22bM. D. Greenhalgh, J. E. Taylor, A. D. Smith, Tetrahedron 2018, 74, 5554–5560.