Synthesis of (±)-Setigerumine I: Biosynthetic Origins of the Elusive Racemic Papaveraceae Isoxazolidine Alkaloids**
Ana V. Serna
Department of Chemistry, Rice University, 6500 Main Street, Houston, TX, 77030 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. László Kürti
Department of Chemistry, Rice University, 6500 Main Street, Houston, TX, 77030 USA
Search for more papers by this authorCorresponding Author
Dr. Juha H. Siitonen
Department of Chemistry, Rice University, 6500 Main Street, Houston, TX, 77030 USA
Search for more papers by this authorAna V. Serna
Department of Chemistry, Rice University, 6500 Main Street, Houston, TX, 77030 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. László Kürti
Department of Chemistry, Rice University, 6500 Main Street, Houston, TX, 77030 USA
Search for more papers by this authorCorresponding Author
Dr. Juha H. Siitonen
Department of Chemistry, Rice University, 6500 Main Street, Houston, TX, 77030 USA
Search for more papers by this authorA previous version of this manuscript has been deposited on a preprint server (https://doi.org/10.33774/chemrxiv-2021-m9ltg).
Graphical Abstract
Abstract
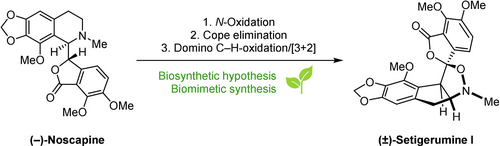
The biosynthetic origins of the structurally related racemic isoxazolidine Papaveraceae alkaloids Setigerumine I, Dactylicapnosinine and Dactylicapnosine have remained elusive since their original isolation over two decades ago. Herein we report the first biosynthetic hypothesis for their formation and, inspired by it, the first synthesis of (±)-Setigerumine I with accompanying computational rationale. Based on the results, these isoxazolidine alkaloids arise from racemizing oxidative rearrangements of prominent isoquinoline alkaloids Noscapine and Hydrastine. The key steps featured in this synthesis are a room temperature Cope elimination and a domino oxidation/inverse-electron demand 1,3-dipolar cycloaddition of an axially chiral, yet configurationally unstable, intermediate. The work opens this previously inaccessible family of natural products for biological studies.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202111049-sup-0001-misc_information.pdf3 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. M. Hagel, P. J. Facchini, Plant Cell Physiol. 2013, 54, 647–672.
- 2
- 2aC. Lee, S. Choe, J. W. Lee, Q. Jin, M. K. Lee, B. Y. Hwang, Bull. Korean Chem. Soc. 2013, 34, 1290–1292;
- 2bG. L. Zhang, G. Rücker, E. Breitmaier, M. Nieger, R. Mayer, C. Steinbeck, Phytochemistry 1995, 40, 299–305.
- 3
- 3aR. C. Godfrey, N. J. Green, G. S. Nichol, A. L. Lawrence, Nat. Chem. 2020, 12, 615–619;
- 3bL. A. M. Murray, S. M. K. McKinnie, H. P. Pepper, R. Erni, Z. D. Miles, M. C. Cruickshank, B. López-Pérez, B. S. Moore, J. H. George, Angew. Chem. Int. Ed. 2018, 57, 11009–11014; Angew. Chem. 2018, 130, 11175–11180;
- 3cB. Yang, G. Wen, Q. Zhang, M. Hou, H. He, S. Gao, J. Am. Chem. Soc. 2021, 143, 6370–6375.
- 4J. Slavík, Czech. Chem. Commun. 1967, 32, 4431–4438.
- 5
- 5aL. Richards, A. Lutz, D. K. Chalmers, A. Jarrold, T. Bowser, G. W. Stevens, S. L. Gras, Biotechnol. Rep. 2019, 24, 1–12;
- 5bL. Richards, A. Jarrold, T. Bowser, G. W. Stevens, S. L. Gras, J. Ind. Microbiol. Biotechnol. 2020, 47, 449–464;
- 5cA. Rosco, H. H. Pauli, W. Priesner, T. M. Kutchan, Arch. Biochem. Biophys. 1997, 348, 369–377;
- 5dT.-D. Nguyen, T.-T. T. Dang, Front. Plant Sci. 2021, 12, 1272.
- 6
- 6aA. Zask, G. Ellestad, Chirality 2018, 30, 157–164;
- 6bA. J. E. Novak, D. Trauner, Trends Chem. 2020, 2, 1052–1065.
- 7
- 7aK. Babanezhad Harikandei, P. Salehi, S. N. Ebrahimi, M. Bararjanian, M. Kaiser, H. R. Khavasi, A. Al-Harrasi, Bioorg. Chem. 2019, 91, 103116;
- 7bS. Aggarwal, N. N. Ghosh, R. Aneja, H. Joshi, R. Chandra, Helv. Chim. Acta 2002, 85, 2458–2462;
- 7cJ. K. Awalt, R. Lam, B. Kellam, B. Graham, P. J. Scammells, R. D. Singer, Green Chem. 2017, 19, 2587–2594;
- 7dA. J. Debono, J. H. Xie, S. Ventura, C. W. Pouton, B. Capuano, P. J. Scammells, ChemMedChem 2012, 7, 2122–2133.
- 8L. Kürti, B. Czakó, Strategic Applications of Named Reactions in Organic Synthesis, Academic Press, Place, 2005.
- 9W. Klötzer, W. E. Oberhänsli, Helv. Chim. Acta 1973, 56, 2107–2110.
- 10
- 10aS. A. Ali, S. M. A. Hashmi, M. N. Siddiqui, M. I. M. Wazeer, Tetrahedron 1996, 52, 14917–14928;
- 10bC. M. Dicken, P. DeShong, J. Org. Chem. 1982, 47, 2047–2051;
- 10cG. W. Gribble, T. C. Barden, J. Org. Chem. 1985, 50, 5900–5902;
- 10dJ. Hamer, A. Macaluso, Chem. Rev. 1964, 64, 473–495.
- 11
- 11aV. Nair, T. D. Suja, Tetrahedron 2007, 63, 12247–12275;
- 11bA. Padwa, Angew. Chem. Int. Ed. Engl. 1976, 15, 123–136; Angew. Chem. 1976, 88, 131–144;
- 11cA. Padwa, W. H. Pearson in Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products (Eds.: Editors ), Wiley, Hoboken, 2002.
10.1002/0471221902 Google Scholar
- 12
- 12aG. Singh, M. P. S. Ishar, V. Gupta, G. Singh, M. Kalyan, S. S. Bhella, Tetrahedron 2007, 63, 4773–4778;
- 12bN. E. Duncan, G. J. Janz, J. Am. Chem. Soc. 1964, 86, 3759–3767;
10.1021/ja01078a085 Google Scholar
- 12cE. Coutouli-Argyropoulou, P. Sarridis, P. Gkizis, Green Chem. 2009, 11, 1906–1914.
- 13
- 13aC. Matassini, C. Parmeggiani, F. Cardona, A. Goti, Org. Lett. 2015, 17, 4082–4085;
- 13bC. Parmeggiani, C. Matassini, F. Cardona, A. Goti, Synthesis 2017, 49, 2890–2900.
- 14
- 14aE. I. Solomon, U. M. Sundaram, T. E. Machonkin, Chem. Rev. 1996, 96, 2563–2605;
- 14bS. D. McCann, S. S. Stahl, Acc. Chem. Res. 2015, 48, 1756–1766.
- 15
- 15aF. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci. 2012, 2, 73–78;
- 15bF. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci. 2018, 8, e1327;
- 15cP. Pracht, F. Bohle, S. Grimme, Phys. Chem. Chem. Phys. 2020, 22, 7169–7192;
- 15dS. Grimme, J. Chem. Theory Comput. 2019, 15, 2847–2862.
- 16
- 16aA. Darcsi, Á. Rácz, S. Béni, J. Pharm. Biomed. Anal. 2017, 134, 187–194;
- 16bA. C. Cope, E. R. Trumbull, Organic Reactions, Wiley, New York, 1960, pp. 317–493;
- 16cR. E. Sammelson, M. J. Kurth, Tetrahedron Lett. 2001, 42, 3419–3422.
- 17K. Hori, J. Ito, T. Ohta, I. Furukawa, Tetrahedron 1998, 54, 12737–12744.
- 18P. P. Painter, R. P. Pemberton, B. M. Wong, K. C. Ho, D. J. Tantillo, J. Org. Chem. 2014, 79, 432–435.
- 19
- 19aH. Kusama, A. Tazawa, K. Ishida, N. Iwasawa, Chem. Asian J. 2016, 11, 64–67;
- 19bR.-Y. Zhu, C.-S. Wang, J. Zheng, F. Shi, S.-J. Tu, J. Org. Chem. 2014, 79, 9305–9312;
- 19cK. B. Simonsen, P. Bayón, R. G. Hazell, K. V. Gothelf, K. A. Jørgensen, J. Am. Chem. Soc. 1999, 121, 3845–3853.
- 20L. R. Domingo, Eur. J. Org. Chem. 2000, 2265–2272.
- 21
- 21aR. Zhang, Q. Guo, E. J. Kennelly, C. Long, X. Chai, Fitoterapia 2020, 146, 104697;
- 21bC. L. Zhang, Q. L. Huang, Q. Zhu, J. Chen, F. Zhang, Z. Y. Cao, Fitoterapia 2020, 144, 104494;
- 21cJ. Répási, S. Hosztafi, Z. Szabó, Planta Med. 1993, 59, 477–478;
- 21dA. F. Aboudi, D. M. Al-Eisawi, S. S. Sabri, M. H. Zarga, J. Nat. Prod. 1986, 49, 370.