Radical 1,4-Aryl Migration Enabled Remote Cross-Electrophile Coupling of α-Amino-β-Bromo Acid Esters with Aryl Bromides
Corresponding Author
Prof. Dr. Shi Tang
College of Chemistry and Chemical Engineering, Jishou University, Jishou, 416000 China
Search for more papers by this authorZhen-Hua Xu
College of Chemistry and Chemical Engineering, Jishou University, Jishou, 416000 China
Search for more papers by this authorTing Liu
College of Chemistry and Chemical Engineering, Jishou University, Jishou, 416000 China
Search for more papers by this authorShuo-Wen Wang
College of Chemistry and Chemical Engineering, Jishou University, Jishou, 416000 China
Search for more papers by this authorJian Yu
College of Chemistry and Chemical Engineering, Jishou University, Jishou, 416000 China
Search for more papers by this authorJian Liu
College of Chemistry and Chemical Engineering, Jishou University, Jishou, 416000 China
Search for more papers by this authorYu Hong
College of Chemistry and Chemical Engineering, Jishou University, Jishou, 416000 China
Search for more papers by this authorProf. Dr. Shi-Lu Chen
Key Laboratory of Cluster Science of Ministry of Education, Beijing Institute of Technology, Beijing, 100081 China
Search for more papers by this authorJin He
College of Chemistry and Chemical Engineering, Jishou University, Jishou, 416000 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Jin-Heng Li
Key Laboratory of Jiangxi Province for Persistent Pollutants Control and Resources Recycle, Nanchang Hangkong University, Nanchang, 330063 China
State Key Laboratory of Chemo/Biosensing and Chemometrics, Hunan University, Changsha, 410082 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Shi Tang
College of Chemistry and Chemical Engineering, Jishou University, Jishou, 416000 China
Search for more papers by this authorZhen-Hua Xu
College of Chemistry and Chemical Engineering, Jishou University, Jishou, 416000 China
Search for more papers by this authorTing Liu
College of Chemistry and Chemical Engineering, Jishou University, Jishou, 416000 China
Search for more papers by this authorShuo-Wen Wang
College of Chemistry and Chemical Engineering, Jishou University, Jishou, 416000 China
Search for more papers by this authorJian Yu
College of Chemistry and Chemical Engineering, Jishou University, Jishou, 416000 China
Search for more papers by this authorJian Liu
College of Chemistry and Chemical Engineering, Jishou University, Jishou, 416000 China
Search for more papers by this authorYu Hong
College of Chemistry and Chemical Engineering, Jishou University, Jishou, 416000 China
Search for more papers by this authorProf. Dr. Shi-Lu Chen
Key Laboratory of Cluster Science of Ministry of Education, Beijing Institute of Technology, Beijing, 100081 China
Search for more papers by this authorJin He
College of Chemistry and Chemical Engineering, Jishou University, Jishou, 416000 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Jin-Heng Li
Key Laboratory of Jiangxi Province for Persistent Pollutants Control and Resources Recycle, Nanchang Hangkong University, Nanchang, 330063 China
State Key Laboratory of Chemo/Biosensing and Chemometrics, Hunan University, Changsha, 410082 China
Search for more papers by this authorGraphical Abstract
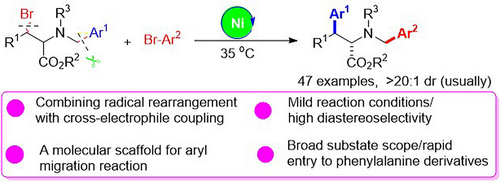
A nickel-catalysed radical relay strategy combines radical rearrangement and cross-coupling via a C(sp3)-centred radical, to produce valuable phenylalanine derivatives that are otherwise challenging to access. An N-benzyl amino acid ester functions as a molecular scaffold for the aryl migration reaction. The synthetic method offers mild reaction conditions, high diastereoselectivity, and broad substrate scope.
Abstract
We report an unprecedented, efficient nickel-catalysed radical relay for the remote cross-electrophile coupling of β-bromo-α-benzylamino acid esters with aryl bromides via 1,4-aryl migration/arylation cascades. β-Bromo-α-benzylamino acid esters are considered as unique molecular scaffolds allowing for aryl migration reactions, which are conceptually novel variants for the radical Truce–Smiles rearrangement. This reaction enables the formation of two new C(sp3)−C(sp2) bonds using a bench-stable Ni/bipyridine/Zn system featuring a broad substrate scope and excellent diastereoselectivity, which provides an effective platform for the remote aryl group migration and arylation of amino acid esters via redox-neutral C(sp3)−C(sp2) bond cleavage. Mechanistically, this cascade reaction is accomplished by combining two powerful catalytic cycles consisting of a cross-electrophile coupling and radical 1,4-aryl migration through the generation of C(sp3)-centred radical intermediates from the homolysis of C(sp3)−Br bonds and the switching of the transient alkyl radical into a robust α-aminoalkyl radical.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202106273-sup-0001-misc_information.pdf21.8 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1a Metal-Catalysed Cross-Coupling Reactions (Eds.: A. de Meijere, F. Diederich), Wiley-VCH, Weinheim, 1998;
- 1b Metal-Catalysed Cross-Coupling Reactions, 2nd ed. (Eds.: A. de Meijere, F. Diederich), Wiley-VCH, Weinheim, 2004.
10.1002/9783527619535 Google Scholar
- 2For selected reviews on cross-electrophile coupling, see:
- 2aD. J. Weix, Acc. Chem. Res. 2015, 48, 1767–1775;
- 2bD. A. Everson, D. J. Weix, J. Org. Chem. 2014, 79, 4793–4798;
- 2cJ.-D. Liu, Y. Ye, L. S. Jonathan, H.-G. Gong, Acc. Chem. Res. 2020, 53, 1833–1845;
- 2dC. E. I. Knappke, S. Grupe, D. Gartner, M. Corpet, C. Gosmini, A. Jacobi von Wangelin, Chem. Eur. J. 2014, 20, 6828–6842;
- 2eT. Moragas, A. Correa, R. Martin, Chem. Eur. J. 2014, 20, 8242–8258;
- 2fX. Wang, Y. Dai, H. Gong, Top. Curr. Chem. 2016, 374, 43.
- 3For selected examples on the construction of C(sp3)-C(sp2) bond via cross-electrophile coupling, see:
- 3aD. A. Everson, R. Shrestha, D. J. Weix, J. Am. Chem. Soc. 2010, 132, 920–921;
- 3bE. C. Hansen, C. Li, S. Yang, D. Pedro, D. J. Weix, J. Org. Chem. 2017, 82, 7085–7089;
- 3cS. Kim, M. J. Goldfogel, M. M. Gilbert, D. J. Weix, J. Am. Chem. Soc. 2020, 142, 9902–9907;
- 3dP. Zhang, C. Le, D. W. C. MacMillan, J. Am. Chem. Soc. 2016, 138, 8084–8087;
- 3eZ.-L. Duan, W. Li, A.-W. Lei, Org. Lett. 2016, 18, 4012–4015;
- 3fX. Wang, G.-B. Ma, Y. Peng, C. E. Pitsch, B. J. Moll, T. D. Ly, X.-T. Wang, H. Gong, J. Am. Chem. Soc. 2018, 140, 14490–14497;
- 3gR. T. Smith, X.-H. Zhang, J. A. Rincon, J. Agejas, C. Mateos, M. Barberis, S. García-Cerrada, O. D. Frutos, J. Am. Chem. Soc. 2018, 140, 17433–17438;
- 3hJ.-C. Duan, Y.-F. Du, X.-B. Pang, X.-Z. Shu, Chem. Sci. 2019, 10, 8706–8712;
- 3iJ.-W. Wang, J.-H. Zhao, H.-G. Gong, Chem. Commun. 2017, 53, 10180–10183.
- 4For selected examples, see:
- 4aE. C. Hansen, D. J. Pedro, A. C. Wotal, N. J. Gower, J. D. Nelson, S. Caron, D. J. Weix, Nat. Chem. 2016, 8, 1126–1130;
- 4bX. Li, Z. Feng, Z.-X. Jiang, X. Zhang, Org. Lett. 2015, 17, 5570–5573;
- 4cV. Krasovskaya, A. Krasovskiy, A. Bhattacharjya, B. H. Lipshutz, Chem. Commun. 2011, 47, 5717–5719;
- 4dA. Krasovskiy, C. Duplais, B. H. Lipshutz, Org. Lett. 2010, 12, 4742–4744;
- 4eC. Qiu, K. Yao, X. Zhang, H. Gong, Org. Biomol. Chem. 2016, 14, 11332–11335;
- 4fN. T. Kadunce, S. E. Reisman, J. Am. Chem. Soc. 2015, 137, 10480–10483.
- 5For selected examples, see:
- 5aG. S. Kumar, A. Peshkov, A. Brzozowska, P. Nikolaienko, C. Zhu, M. Rueping, Angew. Chem. Int. Ed. 2020, 59, 6513–6519; Angew. Chem. 2020, 132, 6575–6581;
- 5bF.-L. Chen, K. Chen, Y. Zhang, Y.-L. He, Y.-M. Wang, S.-L. Zhu, J. Am. Chem. Soc. 2017, 139, 13929–13935;
- 5cY. Li, Y. Luo, L. Peng, Nat. Commun. 2020, 11, 417;
- 5dF. Juliá-Hernández, T. Moragas, J. Cornella, R. Martin, Nature 2017, 545, 84–88.
- 6
- 6aI. Allart-Simon, S. Gérard, J. Sapi, Molecules 2016, 21, 878;
- 6bZ.-M. Chen, X.-M. Zhang, Y.-Q. Tu, Chem. Soc. Rev. 2015, 44, 5220–5245.
- 7
- 7aX.-X. Wu, C. Zhu, Acc. Chem. Res. 2020, 53, 1620–1636;
- 7bY.-L. Chen, L. Chang, Z.-W. Zuo, Acta Chim. Sin. 2019, 77, 794–802.
- 8For selected examples, see:
- 8aN. Wang, Q.-S. Gu, Z.-L. Li, Z. Li, Y.-L. Guo, Z. Guo, X.-Y. Liu, Angew. Chem. Int. Ed. 2018, 57, 14225–14229; Angew. Chem. 2018, 130, 14421–14425;
- 8bN. Zhou, P. Xu, W. Li, Y. Cheng, C. Zhu, Acta Chim. Sin. 2017, 75, 60–65;
- 8cN. Tang, S. Yang, X. Wu, C. Zhu, Tetrahedron 2019, 75, 1639–1646;
- 8dX. Wu, M. Wang, L. Huan, D. Wang, J. Wang, C. Zhu, Angew. Chem. Int. Ed. 2018, 57, 1640–1644; Angew. Chem. 2018, 130, 1656–1660;
- 8eX.-W. Liu, F. Xiong, X.-P. Huang, L. Xu, P.-F. Li, X.-X. Wu, Angew. Chem. Int. Ed. 2013, 52, 6962–6966; Angew. Chem. 2013, 125, 7100–7104;
- 8fB. Sahoo, J.-L. Li, F. Glorius, Angew. Chem. Int. Ed. 2015, 54, 11577–11580; Angew. Chem. 2015, 127, 11740–11744;
- 8gP. Gao, Y.-W. Shen, R. Fang, X.-H. Hao, Z.-H. Qiu, F. Yang, X.-B. Yan, Q. Wang, X.-J. Gong, X.-Y. Liu, Y.-M. Liang, Angew. Chem. Int. Ed. 2014, 53, 7629–7633; Angew. Chem. 2014, 126, 7759–7763;
- 8hL.-Z. Li, P.-J. Cai, Q.-X. Guo, S. Xue, J. Org. Chem. 2008, 73, 3516–3522;
- 8iZ.-M. Chen, W. Bai, S.-H. Wang, B.-M. Yang, Y.-Q. Tu, F.-M. Zhang, Angew. Chem. Int. Ed. 2013, 52, 9781–9785; Angew. Chem. 2013, 125, 9963–9967;
- 8jZ. Wu, D.-P. Wang, Y. Liu, L.-T. Huan, C. Zhu, J. Am. Chem. Soc. 2017, 139, 1388–1391;
- 8kN. Tsuji, Y. Kobayashi, Y. Takemoto, Chem. Commun. 2014, 50, 13691;
- 8lJ. Yan, H. W. Cheo, W. K. Teo, X. Shi, H. Wu, S. B. Idres, L. Deng, J. Wu, J. Am. Chem. Soc. 2020, 142, 11357–11362.
- 9For selected examples, see:
- 9aH.-L. Huang, H. Yan, C. Yang, W. Xia, Chem. Commun. 2015, 51, 4910–4913;
- 9bY. Li, B. Liu, X.-H. Ouyang, R.-J. Song, J.-H. Li, Org. Chem. Front. 2015, 2, 1457–1467;
- 9cS. Tang, L. Yuan, Y.-L. Deng, Z.-Z. Li, L.-N. Wang, G.-X. Huang, R.-L. Sheng, Tetrahedron Lett. 2017, 58, 329–332;
- 9dM. Lu, H. Qin, Z. Lin, M. Huang, W. Weng, S. Cai, Org. Lett. 2018, 20, 7611–7615;
- 9eX.-J. Wei, T. J. Noël, J. Org. Chem. 2018, 83, 11377–11384;
- 9fJ. Liu, S. Wu, J. Yu, C. Lu, Z. Wu, X. Wu, X.-S. Xue, C. Zhu, Angew. Chem. Int. Ed. 2020, 59, 8195–8202; Angew. Chem. 2020, 132, 8272–8279;
- 9gN. Fuentes, W.-Q. Kong, L. Fernandez-Sanchez, E. Merino, C. Nevado, J. Am. Chem. Soc. 2015, 137, 964–973;
- 9hC. M. Holden, S. M. A. Sohel, M. F. Greaney, Angew. Chem. Int. Ed. 2016, 55, 2450–2453; Angew. Chem. 2016, 128, 2496–2499;
- 9iT. M. Monos, R. C. McAtee, C. R. J. Stephenson, Science 2018, 361, 70–74;
- 9jS. Tang, Y.-L. Deng, J. Li, W.-X. Wang, G.-L. Ding, M.-W. Wang, Z.-P. Xiao, Y.-C. Wang, R.-L. Sheng, J. Org. Chem. 2015, 80, 12599–12605;
- 9kZ.-S. Wang, Y.-B. Chen, H.-W. Zhang, Z. Sun, C.-Y. Zhu, L.-W. Ye, J. Am. Chem. Soc. 2020, 142, 3636–3644.
- 10For selected examples, see:
- 10aL. Zhang, G. J. Lovinger, E. K. Edelstein, A. A. Szymaniak, M. P. Chierchia, J. P. Morken, Science 2016, 351, 70–72;
- 10bM. Kischkewitz, K. Okamoto, C. Mück-Lichtenfeld, A. Studer, Science 2017, 355, 936–938;
- 10cG. J. Lovinger, J. P. Morken, J. Am. Chem. Soc. 2017, 139, 17293–17296;
- 10dM. Silvi, C. Sandford, V. K. Aggarwal, J. Am. Chem. Soc. 2017, 139, 5736–5739;
- 10eK. Jana, A. Bhunia, A. Studer, Chem 2020, 6, 512.
- 11For selected examples on aliphatic/aryl amines, see:
- 11aW. Shu, A. Genoux, Z.-D. Li, C. Nevado, Angew. Chem. Int. Ed. 2017, 56, 10521–10524; Angew. Chem. 2017, 129, 10657–10660;
- 11bT.-G. Zhou, F.-X. Luo, M.-Y. Yang, Z.-J. Shi, J. Am. Chem. Soc. 2015, 137, 14586–11458;
- 11cD. Alpers, K. P. Cole, C. R. J. Stephenson, Angew. Chem. Int. Ed. 2018, 57, 12167–12170; Angew. Chem. 2018, 130, 12344–12348;
- 11dY. Wang, J.-X. Zhang, W. Shu, ACS Catal. 2020, 10, 15065–15070.
- 12For selected examples, see:
- 12aZ. Li, M. Wang, Z. Shi, Angew. Chem. Int. Ed. 2021, 60, 186–190; Angew. Chem. 2021, 133, 188–192;
- 12bI. Kim, B. Park, G. Kang, J. Kim, H. Jung, H. Lee, M. H. Baik, S. Hong, Angew. Chem. Int. Ed. 2018, 57, 15517–15522; Angew. Chem. 2018, 130, 15743–15748;
- 12cF. Bu, L. Lu, X. Hu, S. Wang, H. Zhang, A. Lei, Chem. Sci. 2020, 11, 10000–10004;
- 12dJ. Grimshaw, R. Hamilton, J. T. Grimshaw, J. Chem. Soc. Perkin Trans. 1 1982, 229–234;
- 12eS. Amrein, M. Bossart, T. Vasella, A. Studer, J. Org. Chem. 2000, 65, 4281–4288;
- 12fE. Bacqué, M. E. Qacemi, S. Z. Zard, Org. Lett. 2005, 7, 3817–3820.
- 13
- 13aA. C. Durow, G. C. Long, S. J. O'Connel, C. L. Willis, Org. Lett. 2006, 8, 5401–5404;
- 13bG. Cardillo, L. Gentilucci, A. Tolomelli, Mini-Rev. Med. Chem. 2006, 6, 293–304.
- 14For Negishi coupling, see:
- 14aR. F. W. Jackson, N. Wishart, A. Wood, K. James, M. J. Wythes, J. Org. Chem. 1992, 57, 3397–3404;
- 14bP. N. Collier, A. D. Campbell, I. Patel, T. M. Raynham, R. J. K. Taylor, J. Org. Chem. 2002, 67, 1802–1815;
- 14cS. Tang, S. Li, D. Zhou, H. Zeng, N. Wang, Sci. China Chem. 2013, 56, 1293–1300.
- 15M. Sabat, R. C. Johnson, Org. Lett. 2000, 2, 1089–1092.
- 16X. Lu, J. Yi, Z.-Q. Zhang, J.-J. Dai, J.-H. Liu, B. Xiao, Y. Fu, L. Liu, Chem. Eur. J. 2014, 20, 15339–15343.
- 17For C−H arylation, see:
- 17aL. D. Tran, O. Daugulis, Angew. Chem. Int. Ed. 2012, 51, 5188–5191; Angew. Chem. 2012, 124, 5278–5281;
- 17bS.-Y. Zhang, Q. Li, G. He, W. A. Nack, G. Chen, J. Am. Chem. Soc. 2013, 135, 12135–12141;
- 17cG. He, G. Chen, Angew. Chem. Int. Ed. 2011, 50, 5192–5196; Angew. Chem. 2011, 123, 5298–5302;
- 17dJ. He, S.-H. Li, Y.-Q. Deng, H.-Y. Fu, B. N. Laforteza, J. E. Spangler, A. Homs, J.-Q. Yu, Science 2014, 343, 1216–1220.
- 18For selected examples on α-aminoalkyl radical using for cross-coupling:
- 18aA. Remeur, C. B. Kelly, N. R. Patel, G. A. Molander, ACS Catal. 2017, 7, 6065–6069;
- 18bZ.-W. Zuo, D. T. Ahneman, L.-L. Chu, J. A. Terrett, A. G. Doyle, D. W. C. MacMillan, Science 2014, 345, 437–440;
- 18cZ. Zuo, H. Cong, W. Li, J. Choi, G.-C. Fu, D. W. C. MacMillan, J. Am. Chem. Soc. 2016, 138, 1832–1835;
- 18dJ. C. Tellis, D. N. Primer, G. A. Molander, Science 2014, 345, 433–436;
- 18eC. Remeur, C. B. Kelly, N. R. Patel, ACS Catal. 2017, 7, 6065–6069;
- 18fL.-L. Fan, J. Jia, H. Hou, Q. Lefebvre, M. Rueping, Chem. Eur. J. 2016, 22, 16437–16440;
- 18gW.-M. Cheng, R. Sang, Y. Fu, ACS Catal. 2017, 7, 907–911.
- 19For some molecular scaffolds allowing for the aryl migration from alpha-position of N atom to alkyl radical, see:
- 19aJ. Aubé, X. Peng, Y. Wang, F. Takusagawa, J. Am. Chem. Soc. 1992, 114, 5466–5467;
- 19bS.-F. Wang, C.-P. Chuang, J.-H. Lee, S.-T. Liu, Tetrahedron 1999, 55, 2273–2288.
- 20
- 20aF. Bergel, J. A. Stock, J. Chem. Soc. 1954, 2409–2417;
- 20bC.-T. Yen, C.-C. Wu, J.-C. Lee, S.-L. Chen, S. L. Morris-Natschke, P.-W. Hsieh, Y.-C. Wu, Eur. J. Med. Chem. 2010, 45, 2494–2502.
- 21
- 21aJ. Mayol-Llinàs, A. Nelson, W. Farnaby, A. Ayscough, Drug Discovery Today 2017, 22, 965–969;
- 21bV. Lahmy, R. Long, D. Morin, V. Villard, T. Maurice, Front. Cell. Neurosci. 2015, 8, 463;
- 21cA. Trowbridge, D. Reich, M. J. Gaunt, Nature 2018, 561, 522–527.
- 22Deposition Numbers 2072810 (for 3aa), 2072814 (for 3ae), and 2072812 (for 3bg) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
- 23
- 23aC. Zhao, X. Jia, X. Wang, H. Gong, J. Am. Chem. Soc. 2014, 136, 17645–17651;
- 23bL. Huang, L. K. G. Ackerman, K. Kang, A. Parsons, D. J. Weix, J. Am. Chem. Soc. 2019, 141, 10978–10983.
- 24
- 24aS. Condon-Gueugnot, E. Leonel, J.-Y. Nedelec, J. Perichon, J. Org. Chem. 1995, 60, 7684–7686;
- 24bX. Wang, S. Wang, W. Xue, H. Gong, J. Am. Chem. Soc. 2015, 137, 11562–11565.
- 25W. Liu, L. Li, C.-J. Li, Nat. Commun. 2015, 6, 6526.
- 26S. Biswas, D. J. Weix, J. Am. Chem. Soc. 2013, 135, 16192–16197.
- 27
- 27aA. D. Becke, J. Chem. Phys. 1993, 98, 1372;
- 27bP. E. M. Siegbahn, M. R. A. Blomberg, S.-L. Chen, J. Chem. Theory Comput. 2010, 6, 2040–2044.