Copper-Catalyzed and Proton-Directed Selective Hydroxymethylation of Alkynes with CO2
Dr. Mei-Yan Wang
Key Laboratory for Green Chemical Technology of Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Tianjin, 300072 China
Collaborative Innovation Center of Chemical Science and Engineering, Tianjin University, Tianjin, 300072 China
Joint School of National University of Singapore and Tianjin University, International Campus of Tianjin University, Fuzhou, 350207 China
Search for more papers by this authorXin Jin
Key Laboratory for Green Chemical Technology of Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Tianjin, 300072 China
Search for more papers by this authorXiaofei Wang
Key Laboratory for Green Chemical Technology of Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Tianjin, 300072 China
Search for more papers by this authorShumei Xia
State Key Laboratory and Institute of Elemento-Organic Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorYue Wang
Key Laboratory for Green Chemical Technology of Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Tianjin, 300072 China
Search for more papers by this authorShouying Huang
Key Laboratory for Green Chemical Technology of Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Tianjin, 300072 China
Search for more papers by this authorYing Li
Key Laboratory for Green Chemical Technology of Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Tianjin, 300072 China
Search for more papers by this authorCorresponding Author
Prof. Liang-Nian He
State Key Laboratory and Institute of Elemento-Organic Chemistry, Nankai University, Tianjin, 300071 China
Collaborative Innovation Center of Chemical Science and Engineering, Tianjin University, Tianjin, 300072 China
Search for more papers by this authorCorresponding Author
Prof. Xinbin Ma
Key Laboratory for Green Chemical Technology of Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Tianjin, 300072 China
Collaborative Innovation Center of Chemical Science and Engineering, Tianjin University, Tianjin, 300072 China
Joint School of National University of Singapore and Tianjin University, International Campus of Tianjin University, Fuzhou, 350207 China
Search for more papers by this authorDr. Mei-Yan Wang
Key Laboratory for Green Chemical Technology of Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Tianjin, 300072 China
Collaborative Innovation Center of Chemical Science and Engineering, Tianjin University, Tianjin, 300072 China
Joint School of National University of Singapore and Tianjin University, International Campus of Tianjin University, Fuzhou, 350207 China
Search for more papers by this authorXin Jin
Key Laboratory for Green Chemical Technology of Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Tianjin, 300072 China
Search for more papers by this authorXiaofei Wang
Key Laboratory for Green Chemical Technology of Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Tianjin, 300072 China
Search for more papers by this authorShumei Xia
State Key Laboratory and Institute of Elemento-Organic Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorYue Wang
Key Laboratory for Green Chemical Technology of Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Tianjin, 300072 China
Search for more papers by this authorShouying Huang
Key Laboratory for Green Chemical Technology of Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Tianjin, 300072 China
Search for more papers by this authorYing Li
Key Laboratory for Green Chemical Technology of Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Tianjin, 300072 China
Search for more papers by this authorCorresponding Author
Prof. Liang-Nian He
State Key Laboratory and Institute of Elemento-Organic Chemistry, Nankai University, Tianjin, 300071 China
Collaborative Innovation Center of Chemical Science and Engineering, Tianjin University, Tianjin, 300072 China
Search for more papers by this authorCorresponding Author
Prof. Xinbin Ma
Key Laboratory for Green Chemical Technology of Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Tianjin, 300072 China
Collaborative Innovation Center of Chemical Science and Engineering, Tianjin University, Tianjin, 300072 China
Joint School of National University of Singapore and Tianjin University, International Campus of Tianjin University, Fuzhou, 350207 China
Search for more papers by this authorGraphical Abstract
Abstract
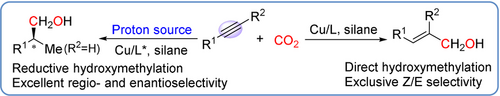
An intriguing strategy for copper-catalyzed hydroxymethylation of alkynes with CO2 and hydrosilane was developed. Switched on/off a proton source, for example, tBuOH, direct hydroxymethylation and reductive hydroxymethylation could be triggered selectively, delivering a series of allylic alcohols and homobenzylic alcohols, respectively, with high levels of Z/E, regio- and enantioselectivity. Such a selective synthesis is attributed to the differences in response of vinylcopper intermediate to proton and CO2. The protonation of vinylcopper species is demonstrated to be prior to hydroxymethylation, thus allowing a diversion from direct alkyne hydroxymethylation to reductive hydroxymethylation in the presence of suitable proton.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202012768-sup-0001-misc_information.pdf5.8 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Selected reviews and examples on CO2 conversion to organic carbonate:
- 1aM. North, R. Pasquale, C. Young, Green Chem. 2010, 12, 1514–1539;
- 1bC. Martín, G. Fiorani, A. W. Kleij, ACS Catal. 2015, 5, 1353–1370;
- 1cB.-H. Xu, J.-Q. Wang, J. Sun, Y. Huang, J.-P. Zhang, X.-P. Zhang, S.-J. Zhang, Green Chem. 2015, 17, 108–122;
- 1dQ. Yang, C.-C. Yang, C.-H. Lin, H.-L. Jiang, Angew. Chem. Int. Ed. 2019, 58, 3511–3515; Angew. Chem. 2019, 131, 3549–3553;
- 1eX. Wang, Y. Zhou, Z. Guo, G. Chen, J. Li, Y. Shi, Y. Liu, J. Wang, Chem. Sci. 2015, 6, 6916–6924;
- 1fL. Wang, G. Zhang, K. Kodama, T. Hirose, Green Chem. 2016, 18, 1229–1233;
- 1gN. Tenhumberg, H. Buttner, B. Schaffner, D. Kruse, M. Blumenstein, T. Werner, Green Chem. 2016, 18, 3775–3788;
- 1hY. A. Rulev, V. A. Larionov, A. V. Lokutova, M. A. Moskalenko, O. L. Lependina, V. I. Maleev, M. North, Y. N. Belokon, ChemSusChem 2016, 9, 216–222.
- 2
- 2aM. Yoshida, N. Hara, S. Okuyama, Chem. Commun. 2000, 151–152;
- 2bM. Xu, A. Jupp, M. Ong, K. Burton, S. Chitnis, D. W. Stephan, Angew. Chem. Int. Ed. 2019, 58, 5707–5711; Angew. Chem. 2019, 131, 5763–5767.
- 3Selected reviews and examples on carboxylation of CO2:
- 3aM. Börjesson, T. Moragas, D. Gallego, R. Martin, ACS Catal. 2016, 6, 6739–6749;
- 3bA. Tortajada, F. Juliá-Hernández, M. Börjesson, T. Moragas, R. Martin, Angew. Chem. Int. Ed. 2018, 57, 15948–15982; Angew. Chem. 2018, 130, 16178–16214;
- 3cJ. Artz, T. E. Müller, K. Thenert, J. Kleinekorte, R. Meys, A. Sternberg, A. Bardow, W. Leitner, Chem. Rev. 2018, 118, 434–504;
- 3dF. Juliá-Hernández, T. Moragas, J. Cornella, R. Martin, Nature 2017, 545, 84–88;
- 3eV. R. Yatham, Y. Shen, R. Martin, Angew. Chem. Int. Ed. 2017, 56, 10915–10919; Angew. Chem. 2017, 129, 11055–11059;
- 3fS. Wang, P. Shao, C. Chen, C. Xi, Org. Lett. 2015, 17, 5112–5115;
- 3gS.-S. Yan, D.-S. Wu, J.-H. Ye, L. Gong, X. Zeng, C.-K. Ran, Y.-Y. Gui, J. Li, D.-G. Yu, ACS Catal. 2019, 9, 6987–6992;
- 3hS.-S. Yan, Q. Fu, L.-L. Liao, G.-Q. Sun, J.-H. Ye, L. Gong, Y.-Z. Bo-Xue, D.-G. Yu, Coord. Chem. Rev. 2018, 374, 439–463.
- 4Examples for CO2 conversion to heterocycle:
- 4aB. Yu, L.-N. He, ChemSusChem 2015, 8, 52–62;
- 4bS. Wang, C. Xi, Chem. Soc. Rev. 2019, 48, 382–404;
- 4cJ.-H. Ye, L. Song, W.-J. Zhou, T. Ju, Z.-B. Yin, S.-S. Yan, Z. Zhang, J. Li, D.-G. Yu, Angew. Chem. Int. Ed. 2016, 55, 10022–10026; Angew. Chem. 2016, 128, 10176–10180;
- 4dT. Kimura, K. Kamata, N. Mizuno, Angew. Chem. Int. Ed. 2012, 51, 6700–6703; Angew. Chem. 2012, 124, 6804–6807;
- 4eJ. Hu, J. Ma, Q. Zhu, Z. Zhang, C. Wu, B. Han, Angew. Chem. Int. Ed. 2015, 54, 5399–5403; Angew. Chem. 2015, 127, 5489–5493;
- 4fM.-Y. Wang, Q.-W. Song, R. Ma, J.-N. Xie, L.-N. He, Green Chem. 2016, 18, 282–287.
- 5Selected reviews and examples for methylation of amines with CO2:
- 5aY. Li, X. Cui, K. Dong, K. Junge, M. Beller, ACS Catal. 2017, 7, 1077–1086;
- 5bY. Markushyna, P. Lamagni, J. Catalano, N. Lock, G. Zhang, M. Antonietti, A. Savateev, ACS Catal. 2020, 10, 7336–7342;
- 5cW. Zhao, X. Chi, H. Li, J. He, J. Long, Y. Xu, S. Yang, Green Chem. 2019, 21, 567–577;
- 5dR. H. Lam, C. M. A. McQueen, I. Pernik, R. T. McBurney, A. F. Hill, B. A. Messerle, Green Chem. 2019, 21, 538–549;
- 5eM.-Y. Wang, N. Wang, X.-F. Liu, C. Qiao, L.-N. He, Green Chem. 2018, 20, 1564–1570;
- 5fH. Niu, L. Lu, R. Shi, C.-W. Chiang, A. Lei, Chem. Commun. 2017, 53, 1148–1151;
- 5gK. Beydoun, T. vom Stein, J. Klankermayer, W. Leitner, Angew. Chem. Int. Ed. 2013, 52, 9554–9557; Angew. Chem. 2013, 125, 9733–9736;
- 5hE. Blondiaux, J. Pouessel, T. Cantat, Angew. Chem. Int. Ed. 2014, 53, 12186–12190; Angew. Chem. 2014, 126, 12382–12386;
- 5iS. Das, F. D. Bobbink, G. Laurenczy, P. J. Dyson, Angew. Chem. Int. Ed. 2014, 53, 12876–12879; Angew. Chem. 2014, 126, 13090–13093.
- 6
- 6aC.-K. Ran, X.-W. Chen, Y.-Y. Gui, J. Liu, L. Song, K. Ren, D.-G. Yu, Sci. China Chem. 2020, 63, 1336–1351;
- 6bN. Kielland, C. J. Whiteoak, A. W. Kleij, Adv. Synth. Catal. 2013, 355, 2115–2138;
- 6cY. Shi, B.-W. Pan, Y. Zhou, J. Zhou, Y.-L. Liu, F. Zhou, Org. Biomol. Chem. 2020, 18, 8597–8619.
- 7
- 7aK.-i. Tominaga, Y. Sasaki, Catal. Commun. 2000, 1, 1–3;
- 7bK.-i. Tominaga, Y. Sasaki, J. Mol. Catal. A 2004, 220, 159–165;
- 7cQ. Liu, L. Wu, I. Fleischer, D. Selent, R. Franke, R. Jackstell, M. Beller, Chem. Eur. J. 2014, 20, 6888–6894.
- 8Y. Tani, K. Kuga, T. Fujihara, J. Terao, Y. Tsuji, Chem. Commun. 2015, 51, 13020–13023.
- 9
- 9aY.-Y. Gui, N. Hu, X.-W. Chen, L. L. Liao, T. Ju, J.-H. Ye, Z. Zhang, J. Li, D.-G. Yu, J. Am. Chem. Soc. 2017, 139, 17011–17014;
- 9bX.-W. Chen, L. Zhu, Y.-Y. Gui, K. Jing, Y.-X. Jiang, Z.-Y. Bo, Y. Lan, J. Li, D.-G. Yu, J. Am. Chem. Soc. 2019, 141, 18825–18835;
- 9cL. Cheng, J. Xie, Chin. J. Org. Chem. 2020, 40, 247–248.
- 10J. Qiu, S. Gao, C. Li, L. Zhang, Z. Wang, X. Wang, K. Ding, Chem. Eur. J. 2019, 25, 13874–13878.
- 11Selected examples for CuH catalyzed reactions:
- 11aB. H. Lipshutz, K. Noson, W. Chrisman, A. Lower, J. Am. Chem. Soc. 2003, 125, 8779–8789;
- 11bA. Saxena, B. Choi, H. W. Lam, J. Am. Chem. Soc. 2012, 134, 8428–8431;
- 11cY. Yang, S.-L. Shi, D. Niu, P. Liu, S. L. Buchwald, Science 2015, 349, 62–66;
- 11dS.-L. Shi, Z. L. Wong, S. L. Buchwald, Nature 2016, 532, 353;
- 11eS. Zhao, N. Mankad, Angew. Chem. Int. Ed. 2018, 57, 5867–5870; Angew. Chem. 2018, 130, 5969–5972;
- 11fY. Xi, J. F. Hartwig, J. Am. Chem. Soc. 2017, 139, 12758–12772;
- 11gS.-L. Shi, S. L. Buchwald, Nat. Chem. 2015, 7, 38.
- 12Selected examples for alkyne semireduction reactions:
- 12aJ. F. Daeuble, C. McGettigan, J. M. Stryker, Tetrahedron Lett. 1990, 31, 2397–2400;
- 12bK. Semba, T. Fujihara, T. Xu, J. Terao, Y. Tsuji, Adv. Synth. Catal. 2012, 354, 1542–1550;
- 12cA. M. Whittaker, G. Lalic, Org. Lett. 2013, 15, 1112–1115; selected examples for addition of olefin-derived nucleophiles to carbonyls:
- 12dY. Yang, I. B. Perry, G. Lu, P. Liu, S. L. Buchwald, Science 2016, 353, 144–150;
- 12eY. Zhou, J. S. Bandar, R. Y. Liu, S. L. Buchwald, J. Am. Chem. Soc. 2018, 140, 606–609.
- 13S. N. Riduan, Y. Zhang, J. Y. Ying, Angew. Chem. Int. Ed. 2009, 48, 3322–3325; Angew. Chem. 2009, 121, 3372–3375.
- 14
- 14aH. Ohmiya, M. Tanabe, M. Sawamura, Org. Lett. 2011, 13, 1086–1088;
- 14bT. Ohishi, M. Nishiura, Z. Hou, Angew. Chem. Int. Ed. 2008, 47, 5792–5795; Angew. Chem. 2008, 120, 5876–5879.
- 15
- 15aM. Hari Babu, G. Ranjith Kumar, R. Kant, M. Sridhar Reddy, Chem. Commun. 2017, 53, 3894–3897;
- 15bT. Fujihara, T. Xu, K. Semba, J. Terao, Y. Tsuji, Angew. Chem. Int. Ed. 2011, 50, 523–527; Angew. Chem. 2011, 123, 543–547.
- 16
- 16aC. Qiao, X.-F. Liu, X. Liu, L.-N. He, Org. Lett. 2017, 19, 1490–1493;
- 16bL. Zhang, J. Cheng, Z. Hou, Chem. Commun. 2013, 49, 4782–4784.
- 17M. W. Gribble, M. T. Pirnot, J. S. Bandar, R. Y. Liu, S. L. Buchwald, J. Am. Chem. Soc. 2017, 139, 2192–2195.
- 18At the current temperature of 65 °C, the protonation of benzylic copper intermediate is slower compared to its carboxylation. Furthermore, the protonation of vinylcopper intermediate consumes most of the tBuOH, further suppressing the protonation of benzylic copper intermediate. For details, see the Supporting Information, Section 3.4.
Citing Literature
February 19, 2021
Pages 3984-3988