Rhodium-Catalyzed Remote C(sp3)−H Borylation of Silyl Enol Ethers
Jie Li
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan, 410082 P. R. China
Search for more papers by this authorProf. Dr. Shuanglin Qu
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan, 410082 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Wanxiang Zhao
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan, 410082 P. R. China
Search for more papers by this authorJie Li
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan, 410082 P. R. China
Search for more papers by this authorProf. Dr. Shuanglin Qu
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan, 410082 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Wanxiang Zhao
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan, 410082 P. R. China
Search for more papers by this authorGraphical Abstract
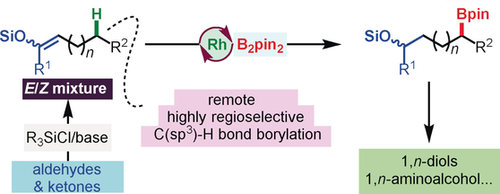
Let's see: A rhodium-catalyzed remote C(sp3)−H borylation of silyl enol ethers (SEEs, E/Z mixtures) by alkene isomerization and hydroboration is reported. This method is compatible with an array of SEEs, including linear and branched SEEs derived from aldehydes and ketones, and provides direct access to a broad range of structurally diverse 1,n-borylethers in excellent regioselectivities and good yields. These compounds are precursors to various valuable chemicals, such as 1,n-diols and aminoalcohols.
Abstract
A rhodium-catalyzed remote C(sp3)−H borylation of silyl enol ethers (SEEs, E/Z mixtures) by alkene isomerization and hydroboration is reported. The reaction exhibits mild reaction conditions and excellent functional-group tolerance. This method is compatible with an array of SEEs, including linear and branched SEEs derived from aldehydes and ketones, and provides direct access to a broad range of structurally diverse 1,n-borylethers in excellent regioselectivities and good yields. These compounds are precursors to various valuable chemicals, such as 1,n-diols and aminoalcohols.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201913281-sup-0001-misc_information.pdf10.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews, see:
- 1aP. B. Reese, Steroids 2001, 66, 481–497;
- 1bI. Franzoni, C. Mazet, Org. Biomol. Chem. 2014, 12, 233–241;
- 1cM. T. Mihai, G. R. Genov, R. J. Phipps, Chem. Soc. Rev. 2018, 47, 149–171;
- 1dA. Dey, S. K. Sinha, T. K. Achar, D. Maiti, Angew. Chem. Int. Ed. 2019, 58, 10820–10843; Angew. Chem. 2019, 131, 10934–10958.
- 2
- 2aA. Vasseur, J. Bruffaerts, I. Marek, Nat. Chem. 2016, 8, 209–219;
- 2bH. Sommer, F. Juliá-Hernández, R. Martin, I. Marek, ACS Cent. Sci. 2018, 4, 153–165;
- 2cD. Janssen-Müller, B. Sahoo, S.-Z. Sun, R. Martin, Isr. J. Chem. 2019, https://doi.org/10.1002/ijch.201900072,
- 2dT. Kochi, S. Kanno, F. Kakiuchi, Tetrahedron Lett. 2019, 60, 150938–150948.
- 3
- 3aE. W. Werner, T.-S. Mei, A. J. Burckle, M. S. Sigman, Science 2012, 338, 1455–1458;
- 3bT.-S. Mei, E. W. Werner, A. J. Burckle, M. S. Sigman, J. Am. Chem. Soc. 2013, 135, 6830–6833;
- 3cT.-S. Mei, H. H. Patel, M. S. Sigman, Nature 2014, 508, 340–344;
- 3dZ.-M. Chen, M. J. Hilton, S. M. Sigman, J. Am. Chem. Soc. 2016, 138, 1146–11464;
- 3eJ. Liu, Q. Yuan, F. D. Toste, M. S. Sigman, Nat. Chem. 2019, 11, 710–715;
- 3fJ. W. Grate, G. C. Frye in Sensors Update, Vol. 2 (Eds.: ), Wiley-VCH, Weinheim, 1996, pp. 10–20.
- 4
- 4aG. C. Fu in Modern Rhodium-Catalyzed Organic Reactions (Ed.: ), Wiley-VCH, Weinheim, 2005, pp. 79–91;
- 4bK. Tanaka, S. Qiao, M. Tobisu, M. M.-C. Lo, G. C. Fu, J. Am. Chem. Soc. 2000, 122, 9870–9871;
- 4cK. Tanaka, G. C. Fu, J. Org. Chem. 2001, 66, 8177–8186;
- 4dN. Chinkov, S. Majumdar, I. Marek, J. Am. Chem. Soc. 2002, 124, 10282–10283;
- 4eN. Chinkov, S. Majumdar, I. Marek, J. Am. Chem. Soc. 2003, 125, 13258–13264;
- 4fA. Masarwa, D. Didier, T. Zabrodski, M. Schinkel, L. Ackermann, I. Marek, Nature 2013, 505, 199–203;
- 4gS. Singh, J. Bruffaerts, A. Vasseur, I. Marek, Nat. Commun. 2017, 8, 14200;
- 4hL. Mantilli, D. Gérard, S. Torche, C. Besnard, C. Mazet, Angew. Chem. Int. Ed. 2009, 48, 5143–5147; Angew. Chem. 2009, 121, 5245–5249;
- 4iE. Larionov, L. Lin, L. Guénée, C. Mazet, J. Am. Chem. Soc. 2014, 136, 16882–16894;
- 4jH. Li, C. Mazet, J. Am. Chem. Soc. 2015, 137, 10720–10727;
- 4kL. Lin, C. Romano, C. Mazet, J. Am. Chem. Soc. 2016, 138, 10344–10350;
- 4lC. Romano, C. Mazet, J. Am. Chem. Soc. 2018, 140, 4743–4750;
- 4mW. N. Palmer, T. Diao, I. Pappas, P. J. Chirik, ACS Catal. 2015, 5, 622–626;
- 4nM. L. Scheuermann, E. J. Johnson, P. J. Chirik, Org. Lett. 2015, 17, 2716–2719;
- 4oJ. V. Obligacion, P. J. Chirik, J. Am. Chem. Soc. 2013, 135, 19107–19110;
- 4pM. Gaydou, T. Moragas, F. Juliá-Hernández, R. Martin, J. Am. Chem. Soc. 2017, 139, 12161–12164;
- 4qF. Juliá-Hernández, T. Moragas, J. Cornella, R. Martin, Nature 2017, 545, 84–88;
- 4rS.-Z. Sun, M. Börjesson, R. Martin-Montero, R. Martin, J. Am. Chem. Soc. 2018, 140, 12765–12769;
- 4sD. B. Grotjahn, C. R. Larsen, J. L. Gustafson, R. Nair, A. Sharma, J. Am. Chem. Soc. 2007, 129, 9592–9593;
- 4tG. Erdogan, D. B. Grotjahn, J. Am. Chem. Soc. 2009, 131, 10354–10355;
- 4uC. R. Larsen, D. B. Grotjahn, J. Am. Chem. Soc. 2012, 134, 10357–10360;
- 4vT.-L. Liu, T. W. Ng, Y. Zhao, J. Am. Chem. Soc. 2017, 139, 3643–3646;
- 4wR.-Z. Huang, K. K. Lau, Z. Li, T.-L. Liu, Y. Zhao, J. Am. Chem. Soc. 2018, 140, 14647–14654;
- 4xF. Chen, K. Chen, Y. Zhang, Y. He, Y.-M. Wang, S. Zhu, J. Am. Chem. Soc. 2017, 139, 13929–13935;
- 4yY. He, Y. Cai, S. Zhu, J. Am. Chem. Soc. 2017, 139, 1061–1064;
- 4zJ. Xiao, Y. He, F. Ye, S. Zhu, Chem 2018, 4, 1645–1657;
- 4aaF. Zhou, J. Zhu, Y. Zhang, S. Zhu, Angew. Chem. Int. Ed. 2018, 57, 4058–4062; Angew. Chem. 2018, 130, 4122–4126;
- 4abY. Zhang, B. Han, S. Zhu, Angew. Chem. Int. Ed. 2019, 58, 13860–13864; Angew. Chem. 2019, 131, 13998–14002;
- 4acY. Zhang, X. Xu, S. Zhu, Nat. Commun. 2019, 10, 1752;
- 4adF. Zhou, Y. Zhang, X. Xu, S. Zhu, Angew. Chem. Int. Ed. 2019, 58, 1754–1758; Angew. Chem. 2019, 131, 1768–1772.
- 5For selected examples, see:
- 5aA. Seayad, M. Ahmed, H. Klein, R. Jackstell, T. Gross, M. Beller, Science 2002, 297, 1676–1678;
- 5bX. Jia, Z. Huang, Nat. Chem. 2015, 8, 157–161;
- 5cY. Ebe, M. Onoda, T. Nishimura, H. Yorimitsu, Angew. Chem. Int. Ed. 2017, 56, 5607–5611; Angew. Chem. 2017, 129, 5699–5703;
- 5dA. J. Borah, Z. Shi, J. Am. Chem. Soc. 2018, 140, 6062–6066;
- 5eT. Kochi, T. Hamasaki, Y. Aoyama, J. Kawasaki, F. Kakiuchi, J. Am. Chem. Soc. 2012, 134, 16544–16547;
- 5fT. Hamasaki, Y. Aoyama, J. Kawasaki, F. Kakiuchi, T. Kochi, J. Am. Chem. Soc. 2015, 137, 16163–16171;
- 5gD. G. Kohler, S. N. Gockel, J. L. Kennemur, P. J. Waller, K. L. Hull, Nat. Chem. 2018, 10, 333–340;
- 5hX. Chen, Z. Cheng, J. Guo, Z. Lu, Nat. Commun. 2018, 9, 3939;
- 5iW. Wang, C. Ding, Y. Li, Z. Li, Y. Li, L. Peng, G. Yin, Angew. Chem. Int. Ed. 2019, 58, 4612–4616; Angew. Chem. 2019, 131, 4660–4664.
- 6There exist only three reports using enelactam or aryl enol ether in chain-walking reactions. See:
- 6aH. H. Patel, M. B. Prater, S. O. Squire, Jr., M. S. Sigman, J. Am. Chem. Soc. 2018, 140, 5895–5898;
- 6bQ. Yuan, M. S. Sigman, J. Am. Chem. Soc. 2018, 140, 6527–6530;
- 6cQ. Yuan, M. S. Sigman, Chem. Eur. J. 2019, 25, 10823–10827.
- 7
- 7aE. Taskinen, J. Chem. Soc. Perkin Trans. 2 2001, 1824–1834;
- 7bR. F. Algera, Y. Ma, D. B. Collum, J. Am. Chem. Soc. 2017, 139, 11544–11549.
- 8
- 8aH. Wakamatsu, M. Nishida, N. Adachi, M. Mori, J. Org. Chem. 2000, 65, 3966–3970;
- 8bC. Su, P. G. Williard, Org. Lett. 2010, 12, 5378–5381;
- 8cE. Larionov, H. Li, C. Mazet, Chem. Commun. 2014, 50, 9816–9826;
- 8dA. L. Kocen, M. Brookhart, O. Daugulis, Chem. Commun. 2017, 53, 10010–10013;
- 8eY. Yamasaki, T. Kumagai, S. Kanno, F. Kakiuchi, T. Kochi, J. Org. Chem. 2018, 83, 9322–9333.
- 9
- 9aS. Kobayashi, K. Manabe, H. Ishitani, J. Matsuo, Science of Synthesis, Vol. 4 (Ed.: ), Thieme, Stuttgart, 1999, chap. 4.16;
- 9b Main Group Metals in Organis Synthesis, Vol. 2 (Eds.: ), Wiley-VCH, Weinheim, 2004, chapter 10.2;
- 9cW. Gati, H. Yamamoto, Acc. Chem. Res. 2016, 49, 1757–1768;
- 9dW. Zhao, J. Sun, Chem. Rev. 2018, 118, 10349–10392.
- 10In the absence of B2pin2, the major side-reaction is the hydrogenation with H2 generated from HBpin (Table 1, entry 14). The role of B2pin2 under the standard reactions conditions is thought to consume the H2 and suppress the hydrogenation. The structure of hydroboration product is shown in the Supporting Information.
- 11C. J. Lata, C. M. Crudden, J. Am. Chem. Soc. 2010, 132, 131–137.
- 12
- 12aS. Shimada, A. S. Batsanov, J. A. K. Howard, T. B. Marder, Angew. Chem. Int. Ed. 2001, 40, 2168–2171;
10.1002/1521-3773(20010601)40:11<2168::AID-ANIE2168>3.0.CO;2-0 CAS PubMed Web of Science® Google ScholarAngew. Chem. 2001, 113, 2226–2229;
- 12bM. A. Esteruelas, M. Oliván, A. Vélez, Organometallics 2015, 34, 1911–1924;
- 12cM. A. Esteruelas, M. Oliván, A. Vélez, J. Am. Chem. Soc. 2015, 137, 12321–12329.
- 13R. Larouche-Gauthier, T. G. Elford, V. K. Aggarwal, J. Am. Chem. Soc. 2011, 133, 16794–16797.
- 14R. P. Sonawane, V. Jheengut, C. Rabalakos, R. Larouche-Gauthier, H. K. Scott, V. K. Aggarwal, Angew. Chem. Int. Ed. 2011, 50, 3760–3763; Angew. Chem. 2011, 123, 3844–3847.
- 15S. N. Mlynarski, C. H. Schuster, J. P. Morken, Nature 2013, 505, 386–390.
- 16E. K. Edelstein, A. C. Grote, M. D. Palkowitz, J. P. Morken, Synlett 2018, 29, 1749–1752.