A Factorial Design Approach for Hydrothermal Synthesis of Phase-Pure AgInO2: A Parametric Optimization Study
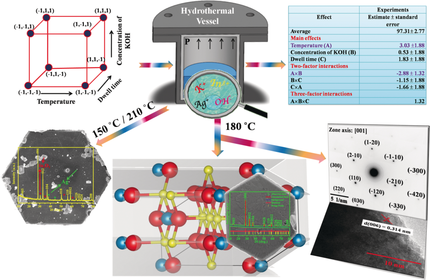
Graphical Abstract
Design! The factorial design approach was adopted for synthesizing phase-pure AgInO2 using a hydrothermal route. Silver nitrate and indium nitrate with KOH as a mineralizer promote the formation of AgInO2 in the form of hexagonal nanoplates. The optimum condition of 180 °C and 4 m KOH was the crucial combination to obtain the pure phase.
Abstract
Owing to a wide range of industrial applications and fundamental importance, delafossite compounds have gathered tremendous interest in research community. In this study, the formation of hexagonal nanoplates of AgInO2 mainly dominated by (00l) facets with no metallic Ag impurity, reported using a facile hydrothermal route at 180 °C using KOH as mineralizer by adopting a factorial design approach. Rietveld analysis of the powder XRD pattern and SAED confirms the rhombohedral system of AgInO2. FE-SEM image shows a uniform hexagonal plate-like morphology with an average width of about 300 nm and thickness of 70 nm. XPS and EDX analysis confirm potassium ion free AgInO2. A specific surface area of about 48.5 m2 g−1 is arrived from N2 adsorption studies. Temperature-dependent AC impedance measurements revealed an activation energy of 0.24 eV/f.u. Further, TG-DTA studies found that the compound is stable in air up to 595 °C.