Reversible Transformation between Amorphous and Crystalline States of Unsaturated Polyesters by Cis–Trans Isomerization
Graphical Abstract
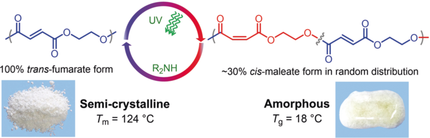
Affairs of state: Aliphatic polyesters prepared from ethylene oxide and maleic anhydride undergo reversible transformation between amorphous and crystalline states through cis–trans isomerization of the C=C bonds in the polymer backbone without any change in either the molecular weight or dispersity of the polymer. A similar transformation was also observed with chiral unsaturated polyesters.
Abstract
An aliphatic polyester has been prepared from ethylene oxide and maleic anhydride that undergoes reversible transformation between amorphous (Tg=18 °C) and crystalline (Tm=124 °C) states through cis–trans isomerization of the C=C bonds in the polymer backbone without any change in either the molecular weight or dispersity of the polymer. A similar transformation was also observed in chiral unsaturated polyesters formed from enantiopure terminal epoxides, such as epichlorohydrin, phenyl glycidyl ether, and (2,3-epoxypropyl)benzene. These unsaturated polyesters with 100 % E-configuration in the crystalline state were prepared by quantitative isomerization of their Z-configuration analogues in the presence of a catalytic amount of diethylamine, while in the presence of benzophenone, irradiation with 365 nm UV light resulted in the transformation of about 30 % trans-alkene to cis-maleate form, thereby affording amorphous polyesters.