Atroposelective Arene Formation by Carbene-Catalyzed Formal [4+2] Cycloaddition
Ke Xu
School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorWenchang Li
School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorShaoheng Zhu
School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Tingshun Zhu
School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorKe Xu
School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorWenchang Li
School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorShaoheng Zhu
School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Tingshun Zhu
School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorGraphical Abstract
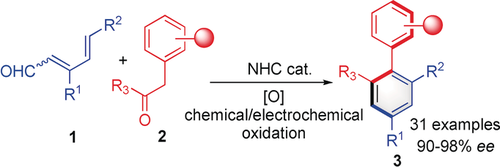
Atroposelective [4+2]: A new organocatalyzed atroposelective arene formation reaction involves a carbene-catalyzed formal [4+2] cycloaddition of conjugated enals and α-aryl ketones. This study expands the synthetic potential of N-heterocyclic carbene (NHC) organocatalysis and provides a competitive pathway for the synthesis of axially chiral ligands, catalysts, and other functional molecules.
Abstract
Atroposelective arene formation is an efficient method to build axially chiral molecules with multi-substituted arenes. Reported here is an organocatalyzed atroposelective arene formation reaction by an N-heterocyclic carbene (NHC) catalyzed formal [4+2] cycloaddition of conjugated dienals and α-aryl ketones. This study expands the synthetic potential of NHC organocatalysis and provides a competitive pathway for the synthesis of axially chiral ligands, catalysts, and other functional molecules.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201910049-sup-0001-misc_information.pdf6.6 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aG. Bringmann, T. Gulder, T. A. M. Gulder, M. Breuning, Chem. Rev. 2011, 111, 563;
- 1bA. Zask, J. Murphy, G. A. Ellestad, Chirality 2013, 25, 265;
- 1cJ. E. Smyth, N. M. Butler, P. A. Keller, Nat. Prod. Rep. 2015, 32, 1562.
- 2
- 2aJ. Clayden, W. J. Moran, P. J. Edwards, S. R. LaPlante, Angew. Chem. Int. Ed. 2009, 48, 6398; Angew. Chem. 2009, 121, 6516;
- 2bS. R. LaPlante, P. J. Edwards, L. D. Fader, A. Jakalian, O. Hucke, ChemMedChem 2011, 6, 505;
- 2cS. R. LaPlante, L. D. Fader, K. R. Fandrick, D. R. Fandrick, O. Hucke, R. Kemper, S. P. F. Miller, P. J. Edwards, J. Med. Chem. 2011, 54, 7005.
- 3
- 3aY. Chen, S. Yekta, A. K. Yudin, Chem. Rev. 2003, 103, 3155;
- 3bH. Shimizu, I. Nagasaki, T. Saito, Tetrahedron 2005, 61, 5405;
- 3cJ. M. Brunel, Chem. Rev. 2005, 105, 857;
- 3dS. Schenker, A. Zamfir, M. Freund, S. B. Tsogoeva, Eur. J. Org. Chem. 2011, 2209.
- 4
- 4aJ. Wencel Delord, A. Panossian, F. R. Lerouxb, F. Colobert, Chem. Soc. Rev. 2015, 44, 3418;
- 4bB. Zilate, A. Castrogiovanni, C. Sparr, ACS Catal. 2018, 8, 2981;
- 4cY.-B. Wang, B. Tan, Acc. Chem. Res. 2018, 51, 534.
- 5Selected reviews:
- 5aM. C. Kozlowski, B. J. Morgan, E. C. Linto, Chem. Soc. Rev. 2009, 38, 3193;
- 5bP. Loxq, E. Manoury, R. Poli, E. Deydier, A. Labande, Coord. Chem. Rev. 2016, 308, 131. Selected recent examples:
- 5cL. Ding, X. Sui, Z. Gu, ACS Catal. 2018, 8, 5630;
- 5dY.-S. Jang, Ł. Wozniak, J. Pedroni, N. Cramer, Angew. Chem. Int. Ed. 2018, 57, 12901; Angew. Chem. 2018, 130, 13083.
- 6
- 6aY.-H. Chen, D.-J. Cheng, J. Zhang, Y. Wang, X.-Y. Liu, B. Tan, J. Am. Chem. Soc. 2015, 137, 15062;
- 6bY.-H. Chen, L.-W. Qi, F. Fang, B. Tan, Angew. Chem. Int. Ed. 2017, 56, 16308; Angew. Chem. 2017, 129, 16526;
- 6cH.-H. Zhang, C.-S. Wang, C. Li, G.-J. Mei, Y. Li, F. Shi, Angew. Chem. Int. Ed. 2017, 56, 116; Angew. Chem. 2017, 129, 122;
- 6dL.-W. Qi, J.-H. Mao, J. Zhang, B. Tan, Nat. Chem. 2018, 10, 58;
- 6eL.-W. Qi, S. Li, S.-H. Xiang, J. Wang, B. Tan, Nat. Catal. 2019, 2, 314.
- 7
- 7aA. I. Meyers, D. G. Wettlaufer, J. Am. Chem. Soc. 1984, 106, 1135;
- 7bR. W. Baker, T. W. Hambley, P. Turner, B. J. Wallace, Chem. Commun. 1996, 2571;
- 7cY. Nishii, K. Wakasugi, K. Koga, Y. Tanabe, J. Am. Chem. Soc. 2004, 126, 5358;
- 7dF. Guo, L. C. Konkol, R. J. Thomson, J. Am. Chem. Soc. 2011, 133, 18;
- 7eV. S. Raut, M. Jean, N. Vanthuyne, C. Roussel, T. Constantieux, C. Bressy, X. Bugaut, D. Bonne, J. Rodriguez, J. Am. Chem. Soc. 2017, 139, 2140;
- 7fA. Link, C. Sparr, Angew. Chem. Int. Ed. 2018, 57, 7136; Angew. Chem. 2018, 130, 7254.
- 8
- 8aR. Miyaji, K. Asano, S. Matsubara, J. Am. Chem. Soc. 2015, 137, 6766;
- 8bG. Liao, B. Li, H.-M. Chen, Q.-J. Yao, Y.-N. Xia, J. Luo, B.-Fe. Shi, Angew. Chem. Int. Ed. 2018, 57, 17151; Angew. Chem. 2018, 130, 17397;
- 8cK. Zhu, K. Xu, Q. Fang, Y. Wang, B. Tang, F. Zhang, ACS Catal. 2019, 9, 4951.
- 9Selected review:
- 9aG. Ma, M. P. Sibi, Chem. Eur. J. 2015, 21, 11644. Selected recent examples:
- 9bJ. L. Gustafson, D. Lim, S. J. Miller, Science 2010, 328, 1251;
- 9cK. Mori, Y. Ichikawa, M. Kobayashi, Y. Shibata, M. Yamanaka, T. Akiyama, J. Am. Chem. Soc. 2013, 135, 3964;
- 9dS. Lu, S. B. Poh, Y. Zhao, Angew. Chem. Int. Ed. 2014, 53, 11041; Angew. Chem. 2014, 126, 11221;
- 9eB. A. Jones, T. Balan, J. D. Jolliffe, C. D. Campbell, M. D. Smith, Angew. Chem. Int. Ed. 2019, 58, 4596; Angew. Chem. 2019, 131, 4644.
- 10
- 10aJ. D. Jolliffe, R. J. Armstrong, M. D. Smith, Nat. Chem. 2017, 9, 558;
- 10bS.-C. Zheng, S. Wu, Q. Zhou, L. W. Chung, L. Ye, B. Tan, Nat. Commun. 2017, 8, 15238;
- 10cS. Jia, Z. Chen, N. Zhang, Y. Tan, Y. Liu, J. Deng, H. Yan, J. Am. Chem. Soc. 2018, 140, 7056.
- 11A. Link, C. Sparr, Chem. Soc. Rev. 2018, 47, 3804.
- 12Selected reviews:
- 12aT. Shibata, K. Tsuchikama, Org. Biomol. Chem. 2008, 6, 1317;
- 12bG. Domínguez, J. Pérez-Castells, Chem. Soc. Rev. 2011, 40, 3430;
- 12cA. Pla-quintana, A. Roglans, Asian J. Org. Chem. 2018, 7, 1706. Selective recent examples:
- 12dT. Shibata, A. Sekine, A. Mitake, K. S. Kanyiva, Angew. Chem. Int. Ed. 2018, 57, 15862; Angew. Chem. 2018, 130, 16088;
- 12eH. Li, X. Yan, J. Zhang, W. Guo, J. Jiang, J. Wang, Angew. Chem. Int. Ed. 2019, 58, 6732; Angew. Chem. 2019, 131, 6804.
- 13
- 13aA. Link, C. Sparr, Angew. Chem. Int. Ed. 2014, 53, 5458; Angew. Chem. 2014, 126, 5562;
- 13bD. Lotter, M. Neuburger, M. Rickhaus, D. Haussinger, C. Sparr, Angew. Chem. Int. Ed. 2016, 55, 2920; Angew. Chem. 2016, 128, 2973;
- 13cV. C. Fäseke, C. Sparr, Angew. Chem. Int. Ed. 2016, 55, 7261; Angew. Chem. 2016, 128, 7378;
- 13dD. Lotter, A. Castrogiovanni, M. Neuburger, C. Sparr, ACS Cent. Sci. 2018, 4, 656.
- 14Selected examples of organocatalytic atroposelective heteroarene formation:
- 14aL. Zhang, J. Zhang, J. Ma, D.-J. Cheng, B. Tan, J. Am. Chem. Soc. 2017, 139, 1714;
- 14bY.-B. Wang, S.-C. Zheng, Y.-M. Hu, B. Tan, Nat. Commun. 2017, 8, 15489;
- 14cY. Liu, X. Wu, S. Li, L. Xue, C. Shan, Z. Zhao, H. Yan, Angew. Chem. Int. Ed. 2018, 57, 6491; Angew. Chem. 2018, 130, 6601;
- 14dC. Zhao, D. Guo, K. Kunkerup, K.-W. Huang, F. Li, J. Wang, Nat. Commun. 2018, 9, 611;
- 14eS.-C. Zheng, Q. Wang, J. Zhu, Angew. Chem. Int. Ed. 2019, 58, 1494; Angew. Chem. 2019, 131, 1508;
- 14fX.-L. He, H.-R. Zhao, X. Song, B. Jiang, W. Du, Y.-C. Chen, ACS Catal. 2019, 9, 4374;
- 14gY. Kwon, J. Li, J. P. Reid, J. M. Crawford, R. Jacob, M. S. Sigman, F. D. Toste, S. J. Miller, J. Am. Chem. Soc. 2019, 141, 6698.
- 15
- 15aStauton–Weireb annulation in total synthesis: C. D. Donner, Tetrahedron 2013, 69, 3747;
- 15bHauser annulation in total synthesis: D. Mal, P. Pahari, Chem. Rev. 2007, 107, 1892;
- 15cR. Peng, M. S. VanNieuwenhze, J. Org. Chem. 2019, 84, 760; 6π-electrocyclization in total synthesis:
- 15dZ. Lu, Y. Li, J. Deng, A. Li, Nat. Chem. 2013, 5, 679;
- 15eJ. Li, P. Yang, M. Yao, J. Deng, A. Li, J. Am. Chem. Soc. 2014, 136, 16477;
- 15fM. Yang, X. Yang, H. Sun, A. Li, Angew. Chem. Int. Ed. 2016, 55, 2851; Angew. Chem. 2016, 128, 2901;
- 15gP. Yang, M. Yao, J. Li, Y. Li, A. Li, Angew. Chem. Int. Ed. 2016, 55, 6964; Angew. Chem. 2016, 128, 7078.
- 16
- 16aM. N. Hopkinson, C. Richter, M. Schedler, F. Glorius, Nature 2014, 510, 485;
- 16bJ. Mahatthananchai, J. W. Bode, Acc. Chem. Res. 2014, 47, 696;
- 16cD. M. Flanigan, F. Romanov-Michailidis, N. A. White, T. Rovis, Chem. Rev. 2015, 115, 9307;
- 16dR. S. Menon, A. T. Biju, V. Nair, Chem. Soc. Rev. 2015, 44, 5040;
- 16eM. H. Wang, K. A. Scheidt, Angew. Chem. Int. Ed. 2016, 55, 14912; Angew. Chem. 2016, 128, 15134;
- 16fC. Zhang, J. F. Hooper, D. W. Lupton, ACS Catal. 2017, 7, 2583;
- 16gS. Mondal, S. R. Yetra, S. Mukherjee, A. T. Biju, Acc. Chem. Res. 2019, 52, 425;
- 16hS. R. Yetra, A. Patra, A. T. Biju, Synthesis 2015, 47, 1357.
- 17
- 17aT. Zhu, P. Zheng, C. Mou, S. Yang, B.-A. Song, Y. R. Chi, Nat. Commun. 2014, 5, 5027;
- 17bT. Zhu, C. Mou, B. Li, M. Smetankova, B.-A. Song, Y. R. Chi, J. Am. Chem. Soc. 2015, 137, 5658;
- 17cX. Huang, T. Zhu, Z. Huang, Y. Zhang, Z. Jin, G. Zanoni, Y. R. Chi, Org. Lett. 2017, 19, 6188.
- 18
- 18aY. Nakano, D. W. Lupton, Angew. Chem. Int. Ed. 2016, 55, 3135; Angew. Chem. 2016, 128, 3187;
- 18bQ. Jia, J. Wang, Org. Lett. 2016, 18, 2212;
- 18cK.-Q. Chen, Z. Luo, Z.-H. Gao, S. Ye, Chem. Eur. J. 2019, 25, 3253.
- 19
- 19aS. De Sarkar, A. Studer, Angew. Chem. Int. Ed. 2010, 49, 9266; Angew. Chem. 2010, 122, 9452;
- 19bS. De Sarkar, S. Grimme, A. Studer, J. Am. Chem. Soc. 2010, 132, 1190.
- 20
- 20aR. M. Gillard, J. E. M. Fernando, D. W. Lupton, Angew. Chem. Int. Ed. 2018, 57, 4712; Angew. Chem. 2018, 130, 4802;
- 20bA. T. Biju, ChemCatChem 2011, 3, 1847.
- 21
- 21aN. Yasmin, J. K. Ray, Synlett 2010, 6, 924;
- 21bR. Singha, S. Dhara, J. K. Ray, Tetrahedron Lett. 2013, 54, 4841.
- 22
- 22aS.-L. Zhang, Z.-L. Yu, J. Org. Chem. 2016, 81, 57;
- 22bR. Bujok, M. Wiszniewski, P. Cmoch, Z. Wróbel, New J. Chem. 2018, 42, 3260.
- 23
- 23aS. S. Sohn, E. L. Rosen, J. W. Bode, J. Am. Chem. Soc. 2004, 126, 14370;
- 23bC. Burstein, F. Glorius, Angew. Chem. Int. Ed. 2004, 43, 6205; Angew. Chem. 2004, 116, 6331.
- 24
- 24aJ. H. Park, S. V. Bhilare, S. W. Youn, Org. Lett. 2011, 13, 2228;
- 24bS. W. Youn, H. S. Song, J. H. Park, Org. Lett. 2014, 16, 1028.
- 25
- 25aM. He, J. R. Struble, J. W. Bode, J. Am. Chem. Soc. 2006, 128, 8418;
- 25bJ. R. Struble, J. W. Bode, Org. Synth. 2010, 87, 362.
- 26
- 26aH. Hénon, M. Mauduit, A. Alexakis, Angew. Chem. Int. Ed. 2008, 47, 9122; Angew. Chem. 2008, 120, 9262;
- 26bM. Tissot, A. P. Hernandez, D. Muller, M. Mauduit, A. Alexakis, Org. Lett. 2011, 13, 1524.
- 27B. A. Bhanu Prasad, S. R. Gilbertson, Org. Lett. 2009, 11, 3710.
- 28
- 28aJ. K. Park, H. H. Lackey, M. D. Rexford, K. Kovnir, M. Shatruk, D. T. McQuade, Org. Lett. 2010, 12, 5008;
- 28bT. N. T. Nguyen, N. O. Thiel, J. F. Teichert, Chem. Commun. 2017, 53, 11686.
- 29
- 29aZ. Zhang, P. R. Schreiner, Chem. Soc. Rev. 2009, 38, 1187;
- 29bX. Fang, C.-J. Wang, Chem. Commun. 2015, 51, 1185;
- 29cY.-L. Sun, Y. Wei, M. Shi, ChemCatChem 2017, 9, 718.
- 30
- 30aS. Bhunia, G. G. Pawar, S. V. Kumar, Y. Jiang, D. Ma, Angew. Chem. Int. Ed. 2017, 56, 16136; Angew. Chem. 2017, 129, 16352;
- 30bJ. Gao, S. Bhunia, K. Wang, L. Gan, S. Xia, D. Ma, Org. Lett. 2017, 19, 2809;
- 30cZ. Chen, Y. Jiang, L. Zhang, Y. Guo, D. Ma, J. Am. Chem. Soc. 2019, 141, 3541.
- 31
- 31aQ. Wang, Q. Gu, S.-L. You, Angew. Chem. Int. Ed. 2019, 58, 6818; Angew. Chem. 2019, 131, 6890;
- 31bJ. Chen, X. Gong, J. Li, Y. Li, J. Ma, C. Hou, G. Zhao, W. Yuan, B. Zhao, Science 2018, 360, 1438.
- 32
- 32aD. S. Surry, S. L. Buchwald, Angew. Chem. Int. Ed. 2008, 47, 6338; Angew. Chem. 2008, 120, 6438;
- 32bJ. M. Dennis, N. A. White, R. Y. Liu, S. L. Buchwald, J. Am. Chem. Soc. 2018, 140, 4721.
- 33
- 33aB. E. Maki, A. Chan, E. M. Phillips, K. A. Scheidt, Org. Lett. 2007, 9, 371;
- 33bB. E. Maki, K. A. Scheidt, Org. Lett. 2008, 10, 4331;
- 33cJ. Mo, R. Yang, X. Chen, B. Tiwari, Y. R. Chi, Org. Lett. 2013, 15, 50.
- 34X. Wu, Y. Zhang, Y. Wang, J. Ke, M. Jeret, R. N. Reddi, S. Yang, B.-A. Song, Y. R. Chi, Angew. Chem. Int. Ed. 2017, 56, 2942; Angew. Chem. 2017, 129, 2988.
- 35
- 35aW. Bauern, D. Nötzel, Ceram. Int. 2014, 40, 4591;
- 35bM. Li, J. Lu, Z. Chen, K. Amine, Adv. Mater. 2018, 30, 1800561.
- 36
- 36aM. Yan, Y. Kawamata, P. S. Baran, Chem. Rev. 2017, 117, 13230;
- 36bY. Jiang, K. Xu, C. Zeng, Chem. Rev. 2018, 118, 4485;
- 36cA. Wiebe, T. Gieshoff, S. Mçhle, E. Rodrigo, M. Zirbes, S. R. Waldvogel, Angew. Chem. Int. Ed. 2018, 57, 5594; Angew. Chem. 2018, 130, 5694;
- 36dN. Sauermann, T. H. Meyer, Y. Qiu, L. Ackermann, ACS Catal. 2018, 8, 7086;
- 36eC. Ma, P. Fang, T.-S. Mei, ACS Catal. 2018, 8, 7179;
- 36fK. D. Moeller, Chem. Rev. 2018, 118, 4817.
- 37CCDC 1954483 (3 d) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.