Ion-Mobility Spectrometry Can Assign Exact Fucosyl Positions in Glycans and Prevent Misinterpretation of Mass-Spectrometry Data After Gas-Phase Rearrangement
Javier Sastre Toraño
Department of Chemical Biology and Drug Discovery, Utrecht University, Universiteitsweg 99, 3584 CG, Utrecht, The Netherlands
Search for more papers by this authorIvan A. Gagarinov
Department of Chemical Biology and Drug Discovery, Utrecht University, Universiteitsweg 99, 3584 CG, Utrecht, The Netherlands
Search for more papers by this authorGaël M. Vos
Department of Chemical Biology and Drug Discovery, Utrecht University, Universiteitsweg 99, 3584 CG, Utrecht, The Netherlands
Search for more papers by this authorFrederik Broszeit
Department of Chemical Biology and Drug Discovery, Utrecht University, Universiteitsweg 99, 3584 CG, Utrecht, The Netherlands
Search for more papers by this authorApoorva D. Srivastava
Department of Chemical Biology and Drug Discovery, Utrecht University, Universiteitsweg 99, 3584 CG, Utrecht, The Netherlands
Search for more papers by this authorDr. Martin Palmer
Waters Corporation, Stamford Avenue, Altrincham Road, SK9 4AX Wilmslow, UK
Search for more papers by this authorDr. James I. Langridge
Waters Corporation, Stamford Avenue, Altrincham Road, SK9 4AX Wilmslow, UK
Search for more papers by this authorDr. Oier Aizpurua-Olaizola
Department of Chemical Biology and Drug Discovery, Utrecht University, Universiteitsweg 99, 3584 CG, Utrecht, The Netherlands
Search for more papers by this authorDr. Victor J. Somovilla
Department of Chemical Biology and Drug Discovery, Utrecht University, Universiteitsweg 99, 3584 CG, Utrecht, The Netherlands
Search for more papers by this authorCorresponding Author
Prof. Dr. Geert-Jan Boons
Department of Chemical Biology and Drug Discovery, Utrecht University, Universiteitsweg 99, 3584 CG, Utrecht, The Netherlands
Complex Carbohydrate Research Center and Department of Chemistry, University of Georgia, 315 Riverbend Road, Athens, GA, 30602 USA
Search for more papers by this authorJavier Sastre Toraño
Department of Chemical Biology and Drug Discovery, Utrecht University, Universiteitsweg 99, 3584 CG, Utrecht, The Netherlands
Search for more papers by this authorIvan A. Gagarinov
Department of Chemical Biology and Drug Discovery, Utrecht University, Universiteitsweg 99, 3584 CG, Utrecht, The Netherlands
Search for more papers by this authorGaël M. Vos
Department of Chemical Biology and Drug Discovery, Utrecht University, Universiteitsweg 99, 3584 CG, Utrecht, The Netherlands
Search for more papers by this authorFrederik Broszeit
Department of Chemical Biology and Drug Discovery, Utrecht University, Universiteitsweg 99, 3584 CG, Utrecht, The Netherlands
Search for more papers by this authorApoorva D. Srivastava
Department of Chemical Biology and Drug Discovery, Utrecht University, Universiteitsweg 99, 3584 CG, Utrecht, The Netherlands
Search for more papers by this authorDr. Martin Palmer
Waters Corporation, Stamford Avenue, Altrincham Road, SK9 4AX Wilmslow, UK
Search for more papers by this authorDr. James I. Langridge
Waters Corporation, Stamford Avenue, Altrincham Road, SK9 4AX Wilmslow, UK
Search for more papers by this authorDr. Oier Aizpurua-Olaizola
Department of Chemical Biology and Drug Discovery, Utrecht University, Universiteitsweg 99, 3584 CG, Utrecht, The Netherlands
Search for more papers by this authorDr. Victor J. Somovilla
Department of Chemical Biology and Drug Discovery, Utrecht University, Universiteitsweg 99, 3584 CG, Utrecht, The Netherlands
Search for more papers by this authorCorresponding Author
Prof. Dr. Geert-Jan Boons
Department of Chemical Biology and Drug Discovery, Utrecht University, Universiteitsweg 99, 3584 CG, Utrecht, The Netherlands
Complex Carbohydrate Research Center and Department of Chemistry, University of Georgia, 315 Riverbend Road, Athens, GA, 30602 USA
Search for more papers by this authorGraphical Abstract
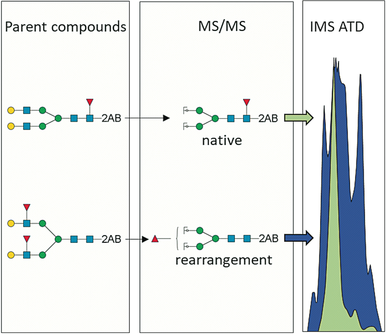
A clear case: The determination of glycan fucosyl positions with MS/MS is complicated due to rearrangements in the gas-phase, leading to erroneous structural assignments. Unique IMS arrival-time distributions of MS/MS fragments can be used to discriminate between fucosides originating from parent compounds and rearranged fucosyl residues, preventing misinterpretation of MS/MS spectra.
Abstract
The fucosylation of glycans leads to diverse structures and is associated with many biological and disease processes. The exact determination of fucoside positions by tandem mass spectrometry (MS/MS) is complicated because rearrangements in the gas phase lead to erroneous structural assignments. Here, we demonstrate that the combined use of ion-mobility MS and well-defined synthetic glycan standards can prevent misinterpretation of MS/MS spectra and incorrect structural assignments of fucosylated glycans. We show that fucosyl residues do not migrate to hydroxyl groups but to acetamido moieties of N-acetylneuraminic acid as well as N-acetylglucosamine residues and nucleophilic sites of an anomeric tag, yielding specific isomeric fragment ions. This mechanistic insight enables the characterization of unique IMS arrival-time distributions of the isomers which can be used to accurately determine fucosyl positions in glycans.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201909623-sup-0001-misc_information.pdf2.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. Varki, Glycobiology 2017, 27, 3–49.
- 2S. Gaunitz, G. Nagy, N. L. B. Pohl, M. V. Noyotny, Anal. Chem. 2017, 89, 389–413.
- 3O. Aizpurua-Olaizola, J. Sastre Toraño, J. M. Falcon-Perez, C. Williams, N. Reichardt, G. J. Boons, TrAC Trends Anal. Chem. 2018, 100, 7–14.
- 4L. Han, C. E. Costello, Biochemistry 2013, 78, 710–720.
- 5B. Küster, T. J. Naven, D. J. Harvey, Rapid Commun. Mass Spectrom. 1996, 10, 1645–1651.
10.1002/(SICI)1097-0231(199610)10:13<1645::AID-RCM664>3.0.CO;2-N CAS PubMed Web of Science® Google Scholar
- 6E. Mucha, M. Lettow, M. Marianski, D. A. Thomas, W. B. Struwe, D. J. Harvey, G. Meijer, P. H. Seeberger, G. von Helden, K. Pagel, Angew. Chem. Int. Ed. 2018, 57, 7440–7443; Angew. Chem. 2018, 130, 7562–7565.
- 7M. Wuhrer, A. M. Deelder, Y. E. van der Burgt, Mass Spectrom. Rev. 2011, 30, 664–680.
- 8J. N. BeMiller, Adv. Carbohydr. Chem. 1967, 22, 25–108.
- 9D. J. Harvey, T. S. Mattu, M. R. Wormald, L. Royle, R. A. Dwek, P. M. Rudd, Anal. Chem. 2002, 74, 734–740.
- 10B. Ernst, D. R. Muller, W. J. Richter, Int. J. Mass Spectrom. Ion Processes 1997, 160, 283–290.
- 11M. Wuhrer, C. A. Koeleman, C. H. Hokke, A. M. Deelder, Rapid Commun. Mass Spectrom. 2006, 20, 1747–1754.
- 12
- 12aY. L. Ma, I. Vedernikova, H. Van den Heuvel, M. Claeys, J. Am. Soc. Mass Spectrom. 2000, 11, 136–144;
- 12bA. H. Franz, C. B. Lebrilla, J. Am. Soc. Mass Spectrom. 2002, 13, 325–337.
- 13J. Li, H.-C. Hsu, J. D. Mountz, J. G. Allen, Cell Chem. Biol. 2018, 25, 499–512.
- 14P.-C. Pang, P. C. N. Chiu, C.-L. Lee, L.-Y. Chang, M. Panico, H. R. Morris, S. M. Haslam, K.-H. Khoo, G. F. Clark, W. S. B. Yeung, A. Dell, Science 2011, 333, 1761–1764.
- 15
- 15aD. J. Becker, J. B. Lowe, Glycobiology 2003, 13, 41R–53R;
- 15bB. Ma, J. L. Simala-Grant, D. E. Taylor, Glycobiology 2006, 16, 158R–184R;
- 15cD. Solter, B. B. Knowles, Proc. Natl. Acad. Sci. USA 1978, 75, 5565–5569;
- 15dT. Muramatsu, H. Muramatsu, Glycoconjugate J. 2004, 21, 41–45.
- 16
- 16aE. Staudacher, F. Altmann, I. B. H. Wilson, L. März, Biochim. Biophys. Acta Gen. Subj. 1999, 1473, 216–236;
- 16bM. Fukuda, Cancer Res. 1996, 56, 2237–2244.
- 17J. Hofmann, K. Pagel, Angew. Chem. Int. Ed. 2017, 56, 8342–8349; Angew. Chem. 2017, 129, 8458–8466.
- 18
- 18aD. J. Harvey, Y. Watanabe, J. D. Allen, P. Rudd, K. Pagel, M. Crispin, W. B. Struwe, J. Am. Soc. Mass Spectrom. 2018, 29, 1250–1261;
- 18bC. J. Gray, B. Thomas, R. Upton, L. G. Migas, C. E. Eyers, P. E. Barran, S. L. Flitsch, Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 1688–1709.
- 19I. A. Gagarinov, T. Li, N. Wei, J. Sastre Torano, R. P. de Vries, M. A. Wolfert, G. J. Boons, Angew. Chem. Int. Ed. 2019, 58, 10547–10552; Angew. Chem. 2019, 131, 10657–10662.
- 20
- 20aC. Manz, K. Pagel, Curr. Opin. Chem. Biol. 2018, 42, 16–24;
- 20bD. J. Harvey, W. B. Struwe, J. Am. Soc. Mass Spectrom. 2018, 29, 1179–1193.
- 21G. Fraenkel, C. Franconi, J. Am. Chem. Soc. 1960, 82, 4478–4483.
- 22L. Liu, A. R. Prudden, C. J. Capicciotti, G. P. Bosman, J. Y. Yang, D. G. Chapla, K. W. Moremen, G. J. Boons, Nat. Chem. 2019, 11, 161–169.
- 23K. Giles, J. Ujma, J. Wildgoose, S. Pringle, K. Richardson, D. Langridge, M. Green, Anal. Chem. 2019, 91, 8564–8573.
- 24P. Both, A. P. Green, C. J. Gray, R. Sardzik, J. Voglmeir, C. Fontana, M. Austeri, M. Rejzek, D. Richardson, R. A. Field, G. Widmalm, S. L. Flitsch, C. E. Eyers, Nat. Chem. 2014, 6, 65–74.
- 25D. T. Kenny, S. M. Issa, N. G. Karlsson, Rapid Commun. Mass Spectrom. 2011, 25, 2611–2618.
- 26C. M. Potel, S. Lemeer, A. J. R. Heck, Anal. Chem. 2019, 91, 126–141.