Metal-Free Synthesis of Benzothiophenes by Twofold C−H Functionalization: Direct Access to Materials-Oriented Heteroaromatics
Dr. Jiajie Yan
School of Chemistry, University of Manchester, Oxford Rd, Manchester, M13 9PL UK
Search for more papers by this authorDr. Alexander P. Pulis
School of Chemistry, University of Manchester, Oxford Rd, Manchester, M13 9PL UK
Search for more papers by this authorDr. Gregory J. P. Perry
School of Chemistry, University of Manchester, Oxford Rd, Manchester, M13 9PL UK
Search for more papers by this authorCorresponding Author
Prof. Dr. David J. Procter
School of Chemistry, University of Manchester, Oxford Rd, Manchester, M13 9PL UK
Search for more papers by this authorDr. Jiajie Yan
School of Chemistry, University of Manchester, Oxford Rd, Manchester, M13 9PL UK
Search for more papers by this authorDr. Alexander P. Pulis
School of Chemistry, University of Manchester, Oxford Rd, Manchester, M13 9PL UK
Search for more papers by this authorDr. Gregory J. P. Perry
School of Chemistry, University of Manchester, Oxford Rd, Manchester, M13 9PL UK
Search for more papers by this authorCorresponding Author
Prof. Dr. David J. Procter
School of Chemistry, University of Manchester, Oxford Rd, Manchester, M13 9PL UK
Search for more papers by this authorGraphical Abstract
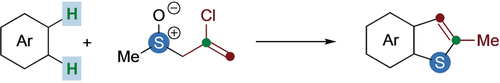
Thio-fusion: Benzothiophenes are accessed from non-prefunctionalized arenes in a one-pot sequence involving an interrupted Pummerer reaction, [3,3]-sigmatropic rearrangement, and cyclization. The process does not require metals, proceeds by twofold C−H functionalization, and can be used to achieve the straightforward π-extension of polyaromatic hydrocarbons.
Abstract
Due to their ubiquity in nature and frequent use in organic electronic materials, benzothiophenes are highly sought after. Here we set out an unprecedented procedure for the formation of benzothiophenes by the twofold vicinal C−H functionalization of arenes that does not require metal catalysis. This one-pot annulation proceeds through an interrupted Pummerer reaction/[3,3]-sigmatropic rearrangement/cyclization sequence to deliver various benzothiophene products. The procedure is particularly effective for the rapid synthesis of benzothiophenes from non-prefunctionalized polyaromatic hydrocarbons (PAHs).
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201908319-sup-0001-misc_information.pdf7.6 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1R. D. Taylor, M. MacCross, A. D. G. Lawson, J. Med. Chem. 2014, 57, 5845–5859.
- 2R. S. Keri, K. Chand, S. Budagumpi, S. Balappa Somappa, S. A. Patil, B. M. Nagaraja, Eur. J. Med. Chem. 2017, 138, 1002–1033.
- 3
- 3aK. Takimiya, S. Shinamura, I. Osaka, E. Miyazaki, Adv. Mater. 2011, 23, 4347–4370;
- 3bW. Jiang, Y. Li, Z. Wang, Chem. Soc. Rev. 2013, 42, 6113–6127;
- 3cC. Wang, H. Dong, W. Hu, Y. Liu, D. Zhu, Chem. Rev. 2012, 112, 2208–2267;
- 3dK. Takimiya, I. Osaka, T. Mori, M. Nakano, Acc. Chem. Res. 2014, 47, 1493–1502;
- 3eM. Stępień, E. Gońka, M. Żyła, N. Sprutta, Chem. Rev. 2017, 117, 3479–3716;
- 3fJ. Chen, K. Yang, X. Zhou, X. Guo, Chem. Asian J. 2018, 13, 2587–2600; Methods for the synthesis of polyaromatic benzothiophenes, related to those in this study, are known, however, they require prefunctionalized arenes and/or multi-step procedures. For prominent examples, see:
- 3gJ. D. Tovar, A. Rose, T. M. Swager, J. Am. Chem. Soc. 2002, 124, 7762–7769;
- 3hL. Meng, T. Fujikawa, M. Kuwayama, Y. Segawa, K. Itami, J. Am. Chem. Soc. 2016, 138, 10351–10355;
- 3iA. Nitti, G. Bianchi, R. Po, T. M. Swager, D. Pasini, J. Am. Chem. Soc. 2017, 139, 8788–8791.
- 4
- 4aD. T. Davies in Aromatic Heterocyclic Chemistry, (Eds.: ), Oxford University Press, New York, 1991;
- 4bJ. A. Joule, K. Mils in Heterocyclic Chemistry, Wiley-Blackwell, Chichester, 2010;
- 4cM. Sainsbury in Heterocyclic Chemistry, The Royal Society of Chemistry, Cambridge, 2001.
- 5
- 5aD. F. Taber, P. K. Tirunahari, Tetrahedron 2011, 67, 7195–7210;
- 5bB. Wu, N. Yoshikai, Org. Biomol. Chem. 2016, 14, 5402–5416.
- 6
- 6aY. Segawa, T. Maekawa, K. Itami, Angew. Chem. Int. Ed. 2015, 54, 66–81; Angew. Chem. 2015, 127, 68–83;
- 6bH. Ito, K. Ozaki, K. Itami, Angew. Chem. Int. Ed. 2017, 56, 11144–11164; Angew. Chem. 2017, 129, 11296–11317;
- 6cH. Ito, Y. Segawa, K. Murakami, K. Itami, J. Am. Chem. Soc. 2019, 141, 3–10.
- 7For select publications, see:
- 7aK. Mochida, K. Kawasumi, Y. Segawa, K. Itami, J. Am. Chem. Soc. 2011, 133, 10716–10719;
- 7bK. Ozaki, K. Kawasumi, M. Shibata, H. Ito, K. Itami, Nat. Commun. 2015, 6, 6251;
- 7cW. Matsuoka, H. Ito, K. Itami, Angew. Chem. Int. Ed. 2017, 56, 12224–12228; Angew. Chem. 2017, 129, 12392–12396;
- 7dY. Yano, N. Mitoma, K. Matsushima, F. Wang, K. Matsui, A. Takakura, Y. Miyauchi, H. Ito, K. Itami, Nature 2019, 571, 387–392.
- 8For select publications, see:
- 8aE. H. Fort, P. M. Donovan, L. T. Scott, J. Am. Chem. Soc. 2009, 131, 16006–16007;
- 8bE. H. Fort, L. T. Scott, Angew. Chem. Int. Ed. 2010, 49, 6626–6628; Angew. Chem. 2010, 122, 6776–6778;
- 8cE. H. Fort, L. T. Scott, Tetrahedron Lett. 2011, 52, 2051–2053.
- 9K. Kawasumi, Q. Zhang, Y. Segawa, L. T. Scott, K. Itami, Nat. Chem. 2013, 5, 739–744.
- 10For select publications, see:
- 10aY.-T. Wu, K.-H. Huang, C.-C. Shin, T.-C. Wu, Chem. Eur. J. 2008, 14, 6697–6703;
- 10bJ. Cai, P. Ruffieux, R. Jaafar, M. Bieri, T. Braun, S. Blankenburg, M. Muoth, A. P. Seitsonen, M. Saleh, X. Feng, K. Müllen, R. Fasel, Nature 2010, 466, 470–473;
- 10cJ. Li, C. Jiao, K.-W. Huang, J. Wu, Chem. Eur. J. 2011, 17, 14672–14680;
- 10dM. V. Pham, N. Cramer, Angew. Chem. Int. Ed. 2014, 53, 3484–3487; Angew. Chem. 2014, 126, 3552–3555;
- 10eF. Xu, X. Xiao, T. R. Hoye, Org. Lett. 2016, 18, 5636–5639.
- 11
- 11aA. P. Pulis, D. J. Procter, Angew. Chem. Int. Ed. 2016, 55, 9842–9860; Angew. Chem. 2016, 128, 9996–10014;
- 11bT. Yanagi, K. Nogi, H. Yorimitsu, Tetrahedron Lett. 2018, 59, 2951–2959.
- 12
- 12aK. Kobayashi, M. Horiuchi, S. Fukamachi, H. Konishi, Tetrahedron 2009, 65, 2430–2435;
- 12bC. Du, S. Ye, J. Chen, Y. Guo, Y. Liu, K. Lu, Y. Liu, T. Qi, X. Gao, Z. Shuai, G. Yu, Chem. Eur. J. 2009, 15, 8275–8282;
- 12cK. Kobayashi, T. Suzuki, M. Horiuchi, Y. Shiroyama, H. Konishi, Synthesis 2011, 2897–2906;
- 12dS. Zhang, X. Qiao, Y. Chen, Y. Wang, R. M. Edkins, Z. Liu, H. Li, Q. Fang, Org. Lett. 2014, 16, 342–345;
- 12eD. Vasu, J. N. Hausmann, H. Saito, T. Yanagi, H. Yorimitsu, A. Osuka, Asian J. Org. Chem. 2017, 6, 1390–1393;
- 12fK. Nobushige, K. Hirano, T. Satoh, M. Miura, Org. Lett. 2014, 16, 1188–1191.
- 13
- 13aS. Yoshida, H. Yorimitsu, K. Oshima, Org. Lett. 2007, 9, 5573–5576;
- 13bS. Otsuka, H. Yorimitsu, A. Osuka, Chem. Eur. J. 2015, 21, 14703–14707.
- 14
- 14aA. J. Eberhart, D. J. Procter, Angew. Chem. Int. Ed. 2013, 52, 4008–4011; Angew. Chem. 2013, 125, 4100–4103;
- 14bA. J. Eberhart, H. J. Shrives, E. Álvarez, A. Carrër, Y. Zhang, D. J. Procter, Chem. Eur. J. 2015, 21, 7428–7434;
- 14cJ. A. Fernández-Salas, A. J. Eberhart, D. J. Procter, J. Am. Chem. Soc. 2016, 138, 790–793;
- 14dA. J. Eberhart, H. Shrives, Y. Zhang, A. Carrër, A. V. S. Parry, D. J. Tate, M. L. Turner, D. J. Procter, Chem. Sci. 2016, 7, 1281–1285.
- 15European Medicines Agency. in ICH Guideline Q3D on Elemental Impurities (London, 2015) http://www.ema.europa.eu/docs/en GB/document library/Scientific guideline/2015/01/WC500180284.pdf.
- 16Ö. Usluer, M. Abbas, G. Wantz, L. Vignau, L. Hirsch, E. Grana, C. Brochon, E. Cloutet, G. Hadziioannou, ACS Macro Lett. 2014, 3, 1134–1138.
- 17K. Takimiya, M. Nakano, M. J. Kang, E. Miyazaki, I. Osaka, Eur. J. Org. Chem. 2013, 217–227.
- 18For a review of the Pummerer reaction, see: L. H. S. Smith, S. C. Coote, H. F. Sneddon, D. J. Procter, Angew. Chem. Int. Ed. 2010, 49, 5832–5844; Angew. Chem. 2010, 122, 5968–5980.
- 19For a review of this subject see
- 19aH. Yorimitsu, Chem. Rec. 2017, 17, 1156–1167; For select publications on intermolecular interrupted Pummerer chemistry with carbon nucleophiles see:
- 19bV. G. Nenajdenko, P. V. Vertelezkij, E. S. Balenkova, Synthesis 1997, 351–355;
- 19cT. Shoji, J. Higashi, S. Ito, K. Toyota, T. Asao, M. Yasunami, K. Fujimori, N. Morita, Eur. J. Org. Chem. 2008, 1242–1252;
- 19dS. Yoshida, H. Yorimitsu, K. Oshima, Org. Lett. 2009, 11, 2185–2188;
- 19eA. J. Eberhart, J. E. Imbriglio, D. J. Procter, Org. Lett. 2011, 13, 5882–5885;
- 19fK. Higuchi, M. Tayu, T. Kawasaki, Chem. Commun. 2011, 47, 6728–6730;
- 19gA. J. Eberhart, C. Cicoira, D. J. Procter, Org. Lett. 2013, 15, 3994–3997;
- 19hX. Huang, M. Patil, C. Farès, W. Thiel, N. Maulide, J. Am. Chem. Soc. 2013, 135, 7312–7323;
- 19iM. Tayu, K. Higuchi, M. Inaba, T. Kawasaki, Org. Biomol. Chem. 2013, 11, 496–502;
- 19jM. Tayu, K. Higuchi, T. Ishizaki, T. Kawasaki, Org. Lett. 2014, 16, 3613–3615;
- 19kM. Tayu, T. Ishizaki, K. Higuchi, T. Kawasaki, Org. Biomol. Chem. 2015, 13, 3863–3865;
- 19lJ. A. Fernández-Salas, A. P. Pulis, D. J. Procter, Chem. Commun. 2016, 52, 12364–12367;
- 19mP. Cowper, Y. Jin, M. D. Turton, G. Kociok-Köhn, S. E. Lewis, Angew. Chem. Int. Ed. 2016, 55, 2564–2568; Angew. Chem. 2016, 128, 2610–2614;
- 19nM. Tayu, Y. Suzuki, K. Higuchi, T. Kawasaki, Synlett 2016, 27, 941–945;
- 19oI. Klose, A. Misale, N. Maulide, J. Org. Chem. 2016, 81, 7201–7210;
- 19pH. J. Shrives, J. A. Fernández-Salas, C. Hedtke, A. P. Pulis, D. J. Procter, Nat. Commun. 2017, 8, 14801;
- 19qM. Tayu, K. Nomura, K. Kawachi, K. Higuchi, N. Saito, T. Kawasaki, Chem. Eur. J. 2017, 23, 10925–10930;
- 19rM. Šiaučiulis, S. Sapmaz, A. P. Pulis, D. J. Procter, Chem. Sci. 2018, 9, 754–759;
- 19sZ. He, H. J. Shrives, J. A. Fernández-Salas, A. Abengózar, J. Neufeld, K. Yang, A. P. Pulis, D. J. Procter, Angew. Chem. Int. Ed. 2018, 57, 5759–5764; Angew. Chem. 2018, 130, 5861–5866;
- 19tG. Hu, J. Xu, P. Li, Org. Chem. Front. 2018, 5, 2167–2170;
- 19uK. Higuchi, T. Tago, Y. Kokubo, M. Ito, M. Tayu, S. Sugiyama, T. Kawasaki, Org. Chem. Front. 2018, 5, 3219–3225;
- 19vB. Waldecker, F. Kraft, C. Golz, M. Alcarazo, Angew. Chem. Int. Ed. 2018, 57, 12538–12542; Angew. Chem. 2018, 130, 12718–12722;
- 19wZ. Zhang, P. He, H. Du, J. Xu, P. Li, J. Org. Chem. 2019, 84, 4517–4524;
- 19xZ. Zhang, Y. Luo, H. Du, J. Xu, P. Li, Chem. Sci. 2019, 10, 5156–5161;
- 19yX. Li, C. Golz, M. Alcarazo, Angew. Chem. Int. Ed. 2019, 58, 9496–9500; Angew. Chem. 2019, 131, 9596–9600;
- 19zM. Šiaučiulis, N. Ahlsten, A. P. Pulis, D. J. Procter, Angew. Chem. Int. Ed. 2019, 58, 8779–8783; Angew. Chem. 2019, 131, 8871–8875.
- 20For example, the well-known trifluoromethylating agent, Umemoto's reagent, is prepared via an intramolecular interrupted Pummerer reaction:
- 20aT. Umemoto, S. Ishihara, Tetrahedron Lett. 1990, 31, 3579–3582;
- 20bT. Umemoto, S. Ishihara, J. Am. Chem. Soc. 1993, 115, 2156–2164.
- 21The X-ray crystallographic data for 3 t, 3 u′, 3 s′, 3 v′ and 3 w′ can be found at the Cambridge Crystallographic Data Centre (CCDC) under deposition numbers CCDC 1934977, 1934978, 1934971, 1947281 and 1934972, respectively.
- 22
- 22aY. Shen, C.-F. Chen, Chem. Rev. 2012, 112, 1463–1535;
- 22b“Thiahelicenes: From Basic Knowledge to Applications”: E. Licandro, S. Cauteruccio, D. Dova in Advances in Heterocyclic Chemistry, Vol. 118, Elsevier, Amsterdam, 2016, pp. 1–46.
- 23K. Takimiya, I. Osaka, Chem. Rec. 2015, 15, 175–188.