Asymmetric Magnesium-Catalyzed Hydroboration by Metal-Ligand Cooperative Catalysis
M. Sc. Alban Falconnet
Institute of Organic Chemistry, RWTH Aachen, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorCorresponding Author
Dr. Marc Magre
Institute of Organic Chemistry, RWTH Aachen, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorCorresponding Author
Dr. Bholanath Maity
KAUST Catalysis Center (KCC), King Abdullah University of Science and Technology (KAUST), Thuwal, 23955-6900 Saudi Arabia
Search for more papers by this authorCorresponding Author
Prof. Dr. Luigi Cavallo
KAUST Catalysis Center (KCC), King Abdullah University of Science and Technology (KAUST), Thuwal, 23955-6900 Saudi Arabia
Search for more papers by this authorCorresponding Author
Prof. Dr. Magnus Rueping
Institute of Organic Chemistry, RWTH Aachen, Landoltweg 1, 52074 Aachen, Germany
KAUST Catalysis Center (KCC), King Abdullah University of Science and Technology (KAUST), Thuwal, 23955-6900 Saudi Arabia
Search for more papers by this authorM. Sc. Alban Falconnet
Institute of Organic Chemistry, RWTH Aachen, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorCorresponding Author
Dr. Marc Magre
Institute of Organic Chemistry, RWTH Aachen, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorCorresponding Author
Dr. Bholanath Maity
KAUST Catalysis Center (KCC), King Abdullah University of Science and Technology (KAUST), Thuwal, 23955-6900 Saudi Arabia
Search for more papers by this authorCorresponding Author
Prof. Dr. Luigi Cavallo
KAUST Catalysis Center (KCC), King Abdullah University of Science and Technology (KAUST), Thuwal, 23955-6900 Saudi Arabia
Search for more papers by this authorCorresponding Author
Prof. Dr. Magnus Rueping
Institute of Organic Chemistry, RWTH Aachen, Landoltweg 1, 52074 Aachen, Germany
KAUST Catalysis Center (KCC), King Abdullah University of Science and Technology (KAUST), Thuwal, 23955-6900 Saudi Arabia
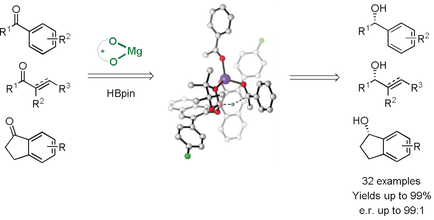
Search for more papers by this authorGraphical Abstract
Mg-ligand cooperative activation of HBpin: The enantioselective magnesium-catalyzed hydroboration of ketones using a (R)-(+)-BINOL derivative as a chiral ligand affords excellent yields and enantioselectivities. Experimental investigations together with DFT calculations provide insight into the reaction mechanism and the origin of enantioselectivity.
Abstract
Asymmetric catalysis with readily available, cheap, and non-toxic alkaline earth metal catalysts represents a sustainable alternative to conventional synthesis methodologies. In this context, we describe the development of a first MgII-catalyzed enantioselective hydroboration providing the products with excellent yields and enantioselectivities. NMR spectroscopy studies and DFT calculations provide insights into the reaction mechanism and the origin of the enantioselectivity which can be explained by a metal-ligand cooperative catalysis pathway involving a non-innocent ligand.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201908012-sup-0001-misc_information.pdf1.9 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1a“MgII and ZnII Lewis acids”: Y. Motoyama, H. Nishiyama, in Lewis Acids in Organic Synthesis (Ed.: ), Wiley-VCH, Weinheim, 2000, pp. 59–88;
10.1002/9783527618309.ch3 Google Scholar
- 1bS. Harder, Alkaline-Earth Metal Compounds: Oddities and Applications, Springer, Berlin, 2013;
10.1007/978-3-642-36270-5 Google Scholar
- 1cM. S. Hill, D. J. Liptrot, C. Weetman, Chem. Soc. Rev. 2016, 45, 972–988.
- 2For recent reviews on the use of organomagnesium catalysts, see:
- 2aM. R. Crimmin, M. S. Hill, Top. Organomet. Chem. 2013, 45, 191–241;
- 2bK. Revunova, G. I. Nikonov, Dalton Trans. 2015, 44, 840–866;
- 2cR. Rochat, M. J. Lopez, H. Tsurugi, K. Mashima, ChemCatChem 2016, 8, 10–20.
- 3For illustrative examples using magnesium organometallics for deprotonation/metalation reactions, see:
- 3aZ. Dong, G. C. Clososki, S. H. Wunderlich, A. Unsinn, J. Li, P. Knochel, Chem. Eur. J. 2009, 15, 457–468;
- 3bF. M. Piller, T. Bresser, M. K. R. Fischer, P. Knochel, J. Org. Chem. 2010, 75, 4365–4375;
- 3cB. Haag, M. Mosrin, H. Ila, V. Malakhov, P. Knochel, Angew. Chem. Int. Ed. 2011, 50, 9794–9824; Angew. Chem. 2011, 123, 9968–9999;
- 3dA. D. Benischke, M. Ellwart, M. R. Becker, P. Knochel, Synthesis 2016, 48, 1101–1107.
- 4Examples for the use of β-diketiminate magnesium complexes in C−H and C−F activations:
- 4aL. Davin, R. McLellan, A. R. Kennedy, E. Hevia, Chem. Commun. 2017, 53, 11650–11653;
- 4bL. Davin, R. McLellan, A. Hernán-Gómez, W. Clegg, A. R. Kennedy, M. Mertens, I. Stepek, E. Hevia, Chem. Commun. 2017, 53, 3653–3656.
- 5Examples using magnesium catalysts for the hydroboration of unsaturated bonds:
- 5aM. Arrowsmith, T. J. Hadlington, M. S. Hill, G. Kociok-Kohn, Chem. Commun. 2012, 48, 4567–4569;
- 5bM. Arrowsmith, M. S. Hill, G. Kociok-Köhn, Chem. Eur. J. 2013, 19, 2776–2783;
- 5cD. Mukherjee, A. Ellern, A. D. Sadow, Chem. Sci. 2014, 5, 959–964;
- 5dL. Fohlmeister, A. Stasch, Chem. Eur. J. 2016, 22, 10235–10246;
- 5eD. Mukherjee, S. Shirase, T. P. Spaniol, K. Mashima, J. Okuda, Chem. Commun. 2016, 52, 13155–13158;
- 5fK. Manna, P. Ji, F. X. Greene, W. Lin, J. Am. Chem. Soc. 2016, 138, 7488–7491;
- 5gM. Rauch, S. Ruccolo, G. Parkin, J. Am. Chem. Soc. 2017, 139, 13264–13267;
- 5hM. Magre, B. Maity, A. Falconnet, J. Cavallo, M. Rueping, Angew. Chem. Int. Ed. 2019, 58, 7025–7029; Angew. Chem. 2019, 131, 7099–7103;
- 5iY. K. Jang, M. Magre, M. Rueping, Org. Lett. 2019, 21, https://doi.org/10.1021/acs.orglett.9b03131 .
- 6For recent reviews on enantioselective magnesium catalysis, see:
- 6aH. Pellissier, Org. Biomol. Chem. 2017, 15, 4750–4782;
- 6bD. Yang, L. Wang, D. Li, R. Wang, Chem 2019, 5, 1108–1166, and references therein.
- 7For representative examples of diols as ligands in combination with magnesium catalysis, see:
- 7aH. Du, X. Zhang, Z. Wang, H. Bao, T. You, K. Ding, Eur. J. Org. Chem. 2008, 2248–2254;
- 7bB. J. Lundy, S. Jansone-Popova, J. A. May, Org. Lett. 2011, 13, 4958–4961;
- 7cB. M. Trost, S. Malhotra, P. Koschker, P. Ellerbrock, J. Am. Chem. Soc. 2012, 134, 2075–2084;
- 7dM. Hatano, T. Horibe, K. Ishihara, Angew. Chem. Int. Ed. 2013, 52, 4549–4553; Angew. Chem. 2013, 125, 4647–4651;
- 7eJ. Zhang, X. Liu, R. Wang, Chem. Eur. J. 2014, 20, 4911–4915;
- 7fD. Li, L. Wang, D. Yang, B. Zhang, R. Wang, ACS Catal. 2015, 5, 7432–7436;
- 7gD. Li, Y. Wang, L. Wang, J. Wang, P. Wang, K. Wang, L. Lin, D. Liu, X. Jiang, D. Yang, Chem. Commun. 2016, 52, 9640–9643;
- 7hD. Li, K. Wang, L. Wang, Y. Wang, P. Wang, X. Liu, D. Yang, R. Wang, Org. Lett. 2017, 19, 3211–3214;
- 7iL. Wang, D. Yang, D. Li, X. Liu, P. Wang, K. Wang, H. Zhu, L. Bai, R. Wang, Angew. Chem. Int. Ed. 2018, 57, 9088–9092; Angew. Chem. 2018, 130, 9226–9230;
- 7jP. Wang, G.-R. Ma, S.-L. Yu, C.-S. Da, Chirality 2019, 31, 79–86.
- 8For cobalt-catalyzed enantioselective hydroboration of ketones, see:
- 8aJ. Guo, J. Chen, Z. Lu, Chem. Commun. 2015, 51, 5725–5727; For manganese-catalyzed enantioselective hydroboration of ketones, see:
- 8bV. Vasilenko, C. K. Blasius, H. Wadepohl, L. H. Gade, Angew. Chem. Int. Ed. 2017, 56, 8393–8397; Angew. Chem. 2017, 129, 8513–8517;
- 8cV. Vasilenko, C. K. Blasius, L. H. Gade, J. Am. Chem. Soc. 2018, 140, 9244–9254; For nickel-catalyzed enantioselective hydroboration of α,β-unsaturated ketones, see:
- 8dF. Chen, Y. Zhang, L. Yu, S. Zhu, Angew. Chem. Int. Ed. 2017, 56, 2022–2025; Angew. Chem. 2017, 129, 2054–2057; For rare-earth metals catalyzed hydroboration of ketones and α,β-unsaturated ketones, see:
- 8eP. Song, C. Lu, Z. Fei, B. Zhao, Y. Yao, J. Org. Chem. 2018, 83, 6093–6100; For a review on enantioselective hydroboration of ketones, see:
- 8fB. T. Cho, Chem. Soc. Rev. 2009, 38, 443–452; Leading examples:
- 8gA. Hirao, S. Itsuno, S. Nakahama, N. Yamazaki, Chem. Commun. 1981, 315–317;
- 8hS. Itsuno, M. Nakano, K. Miyazaki, H. Masuda, K. Ito, A. Hirao, S. Nakahama, J. Chem. Soc. 1985, 2039–2044;
- 8iE. J. Corey, R. K. Bakshi, S. Shibata, J. Am. Chem. Soc. 1987, 109, 5551–5553;
- 8jE. J. Corey, R. K. Bakshi, S. Shibata, C. P. Chen, V. K. Singh, J. Am. Chem. Soc. 1987, 109, 7925–7926.
- 9Enantioselective hydroboration of propargylic ketones:
- 9aUsing phosphoric acid as catalyst and HCat as hydroborating agent: Z. Zhang, P. Jain, J. C. Antilla, Angew. Chem. Int. Ed. 2011, 50, 10961–10964; Angew. Chem. 2011, 123, 11153–11156; In this case, 3 aa was isolated in 12 % ee.
- 9bUsing manganese(II) as catalyst and HBpin as reducing agent, see: Ref. [8b]. In this case, 3 aa was isolated in 86 % ee. For enantioselective hydroboration of 2 ae using chiral oxazoborolidines. See:
- 9cC. J. Helal, P. A. Magriotis, E. J. Corey, J. Am. Chem. Soc. 1996, 118, 10938–10939.
- 10Usually the range of Mg−H in 1H NMR spectra ([D8]toluene or [D8]THF) is between δ=4.1–3.5 ppm. See for example:
- 10aS. J. Bonyhady, C. Jones, S. Nembenna, A. Stasch, A. J. Edwards, G. J. McIntyre, Chem. Eur. J. 2010, 16, 938–955;
- 10bM. Arrowsmith, B. Maitland, G. Kociok-Köhn, A. Stasch, C. Jones, M. S. Hill, Inorg. Chem. 2014, 53, 10543–10552;
- 10cR. Lalrempuia, A. Stasch, C. Jones, Chem. Asian J. 2015, 10, 447–454;
- 10dS. Schnitzler, T. P. Spaniol, L. Maron, J. Okuda, Chem. Eur. J. 2015, 21, 11330–11334;
- 10eS. Schnitzler, T. P. Spaniol, J. Okuda, Inorg. Chem. 2016, 55, 12997–13006;
- 10fM. J. Butler, M. R. Crimmin, Chem. Commun. 2017, 53, 1348–1365, and references therein.
- 11For some Mg−B 11B NMR references in the literature, see:
- 11aM. Yamashita, Y. Suzuki, Y. Segawa, K. Nozaki, J. Am. Chem. Soc. 2007, 129, 9570–9571;
- 11bA.-F. Pécharman, A. L. Colebatch, M. S. Hill, C. L. McMullin, M. F. Mahon, C. Weetman, Nat. Commun. 2017, 8, 15022;
- 11cA.-F. Pécharman, M. S. Hill, C. L. McMullin, M. F. Mahon, Angew. Chem. Int. Ed. 2017, 56, 16363–16366; Angew. Chem. 2017, 129, 16581–16584;
- 11dA.-F. Pécharman, M. S. Hill, M. F. Mahon, Dalton Trans. 2018, 47, 7300–7305.
- 12Tripodal aluminum complex activated HBpin through ligand–boron interaction, increasing the nucleophilicity of the hydride. In this case, no Al−H species were observed. A. J. Woodside, M. A. Smith, T. M. Herb, B. C. Manor, P. J. Carrol, P. R. Rablen, C. R. Graves, Organometallics 2019, 38, 1017–1020.