Functionalized Contorted Polycyclic Aromatic Hydrocarbons by a One-Step Cyclopentannulation and Regioselective Triflyloxylation
M. Sc. Xuan Yang
Organisch-Chemisches Institut, Ruprecht-Karls-Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Search for more papers by this authorM. Sc. Marvin Hoffmann
Theoretical and Computational Chemistry, Interdisciplinary Center for Scientific Computing (IWR), Ruprecht-Karls- Universität Heidelberg, Im Neuenheimer Feld 205A, 69120 Heidelberg, Germany
Search for more papers by this authorDr. Frank Rominger
Organisch-Chemisches Institut, Ruprecht-Karls-Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Search for more papers by this authorM. Sc. Tobias Kirschbaum
Organisch-Chemisches Institut, Ruprecht-Karls-Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Search for more papers by this authorProf. Dr. Andreas Dreuw
Theoretical and Computational Chemistry, Interdisciplinary Center for Scientific Computing (IWR), Ruprecht-Karls- Universität Heidelberg, Im Neuenheimer Feld 205A, 69120 Heidelberg, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Michael Mastalerz
Organisch-Chemisches Institut, Ruprecht-Karls-Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Search for more papers by this authorM. Sc. Xuan Yang
Organisch-Chemisches Institut, Ruprecht-Karls-Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Search for more papers by this authorM. Sc. Marvin Hoffmann
Theoretical and Computational Chemistry, Interdisciplinary Center for Scientific Computing (IWR), Ruprecht-Karls- Universität Heidelberg, Im Neuenheimer Feld 205A, 69120 Heidelberg, Germany
Search for more papers by this authorDr. Frank Rominger
Organisch-Chemisches Institut, Ruprecht-Karls-Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Search for more papers by this authorM. Sc. Tobias Kirschbaum
Organisch-Chemisches Institut, Ruprecht-Karls-Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Search for more papers by this authorProf. Dr. Andreas Dreuw
Theoretical and Computational Chemistry, Interdisciplinary Center for Scientific Computing (IWR), Ruprecht-Karls- Universität Heidelberg, Im Neuenheimer Feld 205A, 69120 Heidelberg, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Michael Mastalerz
Organisch-Chemisches Institut, Ruprecht-Karls-Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Search for more papers by this authorGraphical Abstract
Abstract
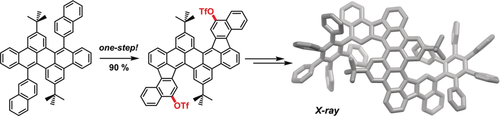
The oxidative cyclodehydrogenation (often named the Scholl reaction) is still a powerful synthetic tool to construct even larger polycyclic aromatic hydrocarbons (PAHs) by multiple biaryl bond formations without the necessity of prior installation of reacting functional groups. Scholl-type reactions are usually very selective although the resulting products bear sometimes some surprises, such as the formation of five-membered instead of six-membered rings or the unexpected migration of aryl moieties. There are a few examples, where chlorinated byproducts were found when FeCl3 was used as reagent. To our knowledge, the direct functionalization of PAHs during Scholl-type cyclization by triflyloxylation has not been observed. Herein we describe the synthesis of functionalized PAHs by the formation of five-membered rings and a regioselective triflyloxylation in one step. The triflyloxylated PAHs can be used as reactants for further transformation to even larger contorted PAHs.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201905666-sup-0001-misc_information.pdf9.5 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aY. Segawa, H. Ito, K. Itami, Nat. Rev. Mater. 2016, 1, 15002;
- 1bJ. C. Fetzer, W. R. Biggs, Polycyclic Aromat. Compd. 1994, 4, 3–17;
- 1cM. Müller, C. Kübel, K. Müllen, Chem. Eur. J. 1998, 4, 2099–2109;
10.1002/(SICI)1521-3765(19981102)4:11<2099::AID-CHEM2099>3.0.CO;2-T CAS Web of Science® Google Scholar
- 1dJ. Wu, W. Pisula, K. Müllen, Chem. Rev. 2007, 107, 718–747;
- 1eK. Müllen, ACS Nano 2014, 8, 6531–6541;
- 1fA. Narita, X.-Y. Wang, X. Feng, K. Müllen, Chem. Soc. Rev. 2015, 44, 6616–6643.
- 2O. V. Yazyev, Acc. Chem. Res. 2013, 46, 2319–2328.
- 3
- 3aK. Baumgärtner, F. Rominger, M. Mastalerz, Chem. Eur. J. 2018, 24, 8751–8755;
- 3bR. Yamaguchi, S. Hiroto, H. Shinokubo, Org. Lett. 2012, 14, 2472–2475;
- 3cR. Yamaguchi, S. Ito, B. S. Lee, S. Hiroto, D. Kim, H. Shinokubo, Chem. Asian J. 2013, 8, 178–190.
- 4
- 4aM. Grzybowski, K. Skonieczny, H. Butenschön, D. T. Gryko, Angew. Chem. Int. Ed. 2013, 52, 9900–9930; Angew. Chem. 2013, 125, 10084–10115;
- 4bR. Scholl, C. Seer, R. Weitzenböck, Ber. Dtsch. Chem. Ges. 1910, 43, 2202–2209.
- 5
- 5aY. Avlasevich, C. Kohl, K. Müllen, J. Mater. Chem. 2006, 16, 1053–1057;
- 5bK. Ota, T. Tanaka, A. Osuka, Org. Lett. 2014, 16, 2974–2977;
- 5cChaolumen, M. Murata, Y. Sugano, A. Wakamiya, Y. Murata, Angew. Chem. Int. Ed. 2015, 54, 9308–9312; Angew. Chem. 2015, 127, 9440–9444;
- 5dA. Naibi Lakshminarayana, J. Chang, J. Luo, B. Zheng, K.-W. Huang, C. Chi, Chem. Commun. 2015, 51, 3604–3607;
- 5eS. Kumar, M.-T. Ho, Y.-T. Tao, Org. Lett. 2016, 18, 200–203;
- 5fJ. Liu, A. Narita, S. Osella, W. Zhang, D. Schollmeyer, D. Beljonne, X. Feng, K. Müllen, J. Am. Chem. Soc. 2016, 138, 2602–2608;
- 5gJ. Liu, S. Osella, J. Ma, R. Berger, D. Beljonne, D. Schollmeyer, X. Feng, K. Müllen, J. Am. Chem. Soc. 2016, 138, 8364–8367;
- 5hChaolumen, M. Murata, A. Wakamiya, Y. Murata, Angew. Chem. Int. Ed. 2017, 56, 5082–5086; Angew. Chem. 2017, 129, 5164–5168;
- 5iS. Nobusue, K. Fujita, Y. Tobe, Org. Lett. 2017, 19, 3227–3230;
- 5jM. Kawamura, E. Tsurumaki, S. Toyota, Synthesis 2018, 02, 134–138;
- 5kL. J. Purvis, X. Gu, S. Ghosh, Z. Zhang, C. J. Cramer, C. J. Douglas, J. Org. Chem. 2018, 83, 1828–1841.
- 6
- 6aA. Pradhan, P. Dechambenoit, H. Bock, F. Durola, J. Org. Chem. 2013, 78, 2266–2274;
- 6bJ. M. Fernández-García, P. J. Evans, S. Medina Rivero, I. Fernández, D. García-Fresnadillo, J. Perles, J. Casado, N. Martín, J. Am. Chem. Soc. 2018, 140, 17188–17196;
- 6cK. Kawasumi, Q. Zhang, Y. Segawa, L. T. Scott, K. Itami, Nat. Chem. 2013, 5, 739.
- 7
- 7aY. Sakamoto, T. Suzuki, J. Am. Chem. Soc. 2013, 135, 14074–14077;
- 7bK. Y. Cheung, C. K. Chan, Z. Liu, Q. Miao, Angew. Chem. Int. Ed. 2017, 56, 9003–9007; Angew. Chem. 2017, 129, 9131–9135.
- 8
- 8aY. N. Oded, S. Pogodin, I. Agranat, J. Org. Chem. 2016, 81, 11389–11393;
- 8bM. S. Markoulides, C. Venturini, D. Neumeyer, A. Gourdon, New J. Chem. 2015, 39, 6498–6503.
- 9
- 9aH. Shinokubo, Proc. Jpn. Acad. Ser. B 2014, 90, 1–11;
- 9bG. Li, H. Phan, T. S. Herng, T. Y. Gopalakrishna, C. Liu, W. Zeng, J. Ding, J. Wu, Angew. Chem. Int. Ed. 2017, 56, 5012–5016; Angew. Chem. 2017, 129, 5094–5098;
- 9cQ. Chen, D. Wang, M. Baumgarten, D. Schollmeyer, K. Müllen, A. Narita, Chem. Asian J. 2019, 14, 1703–1707.
- 10Y.-Z. Tan, B. Yang, K. Parvez, A. Narita, S. Osella, D. Beljonne, X. Feng, K. Müllen, Nat. Commun. 2013, 4, 2646.
- 11P. Sarkar, P. Dechambenoit, F. Durola, H. Bock, Asian J. Org. Chem. 2012, 1, 366–376.
- 12Y. Yang, L. Yuan, B. Shan, Z. Liu, Q. Miao, Chem. Eur. J. 2016, 22, 18620–18627.
- 13
- 13aA. Pradhan, P. Dechambenoit, H. Bock, F. Durola, Angew. Chem. Int. Ed. 2011, 50, 12582–12585; Angew. Chem. 2011, 123, 12790–12793;
- 13bM. S. Little, S. G. Yeates, A. A. Alwattar, K. W. J. Heard, J. Raftery, A. C. Edwards, A. V. S. Parry, P. Quayle, Eur. J. Org. Chem. 2017, 1694–1703.
- 14
- 14aE. A. Bliss, R. J. Griffin, M. F. G. Stevens, J. Chem. Soc. Perkin Trans. 1 1987, 2217–2228;
- 14bR. A. Abramovitch, P. Chinnasamy, K. Evertz, G. Huttner, J. Chem. Soc. Chem. Commun. 1989, 3–5;
- 14cM. A. Castro, M. F. G. Stevens, J. Chem. Soc. Chem. Commun. 1992, 869–870.
- 15G. Zhang, F. Rominger, U. Zschieschang, H. Klauk, M. Mastalerz, Chem. Eur. J. 2016, 22, 14840–14845.
- 16X. Yang, F. Rominger, M. Mastalerz, Org. Lett. 2018, 20, 7270–7273.
- 17
- 17aK. Shiraishi, A. Rajca, M. Pink, S. Rajca, J. Am. Chem. Soc. 2005, 127, 9312–9313;
- 17bT. Fujikawa, Y. Segawa, K. Itami, J. Am. Chem. Soc. 2015, 137, 7763–7768;
- 17cV. Berezhnaia, M. Roy, N. Vanthuyne, M. Villa, J.-V. Naubron, J. Rodriguez, Y. Coquerel, M. Gingras, J. Am. Chem. Soc. 2017, 139, 18508–18511;
- 17dY. Zhu, Z. Xia, Z. Cai, Z. Yuan, N. Jiang, T. Li, Y. Wang, X. Guo, Z. Li, S. Ma, D. Zhong, Y. Li, J. Wang, J. Am. Chem. Soc. 2018, 140, 4222–4226.
- 18
- 18aB. Panda, T. K. Sarkar, Tetrahedron Lett. 2010, 51, 301–305;
- 18bJ. Cao, M. Miao, W. Chen, L. Wu, X. Huang, J. Org. Chem. 2011, 76, 9329–9337.
- 19
- 19aW. Dilthey, G. Hurtig, Ber. Dtsch. Chem. Ges. B 1934, 67, 495–496;
- 19bY. Zhu, X. Guo, Y. Li, J. Wang, J. Am. Chem. Soc. 2019, 141, 5511–5517.
- 20CCDC 1914188 (4), 1914189 (7), and 1914190 (15) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.