A Regio- and Stereodivergent Synthesis of Homoallylic Amines by a One-Pot Cooperative-Catalysis-Based Allylic Alkylation/Hofmann Rearrangement Strategy
Dr. Colin M. Pearson
Department of Chemistry, Indiana University, 800 East Kirkwood Avenue, Bloomington, IN, 47405 USA
These authors contributed equally to this work.
Search for more papers by this authorDr. James W. B. Fyfe
Department of Chemistry, Indiana University, 800 East Kirkwood Avenue, Bloomington, IN, 47405 USA
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Prof. Thomas N. Snaddon
Department of Chemistry, Indiana University, 800 East Kirkwood Avenue, Bloomington, IN, 47405 USA
Search for more papers by this authorDr. Colin M. Pearson
Department of Chemistry, Indiana University, 800 East Kirkwood Avenue, Bloomington, IN, 47405 USA
These authors contributed equally to this work.
Search for more papers by this authorDr. James W. B. Fyfe
Department of Chemistry, Indiana University, 800 East Kirkwood Avenue, Bloomington, IN, 47405 USA
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Prof. Thomas N. Snaddon
Department of Chemistry, Indiana University, 800 East Kirkwood Avenue, Bloomington, IN, 47405 USA
Search for more papers by this authorDedicated to Professor Alois Fürstner
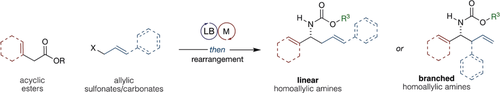
Graphical Abstract
Cooperation then amination: A unified one-pot experimental procedure enables a versatile regio- and stereodivergent synthesis of homoallylic amines. Critical to the successful development of this method was the recognition that initial catalyzed C−C bond formation controls all aspects of regio- and stereoselectivity. Thereafter, in situ amination/Hofmann rearrangement results in stereospecific C−N bond formation.
Abstract
Herein, we report a modular synthetic route to linear and branched homoallylic amines that operates through a sequential one-pot Lewis base/transition-metal catalyzed allylic alkylation/Hofmann rearrangement strategy. This protocol is operationally trivial, proceeds from simple and easily prepared substrates and catalysts, and enables all aspects of regio- and stereoselectivity to be controlled through a conserved experimental protocol. Overall, the high levels of enantio-, regio-, and diastereoselectivity obtained, in concert with the ability to access orthogonally protected or free amines, render this a straightforward and effective approach for the preparation of useful enantioenriched homoallylic amines. We have also demonstrated the utility of the products in the context of pharmaceutical synthesis.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201905426-sup-0001-misc_information.pdf8.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1S. A. Lawrence, Amines: Synthesis, Properties and Applications, Cambridge University Press, Cambridge, 2004.
- 2B. Weiner, W. Szymański, D. B. Janssen, A. J. Minnaard, B. L. Feringa, Chem. Soc. Rev. 2010, 39, 1656–1691.
- 3For reviews, see:
- 3aM. Yus, J. C. González-Gómez, F. Foubelo, Chem. Rev. 2013, 113, 5595–5698;
- 3bM. Yus, J. C. González-Gómez, F. Foubelo, Chem. Rev. 2011, 111, 7774–7854;
- 3cS. Kobayashi, Y. Mori, J. S. Fossey, M. M. Salter, Chem. Rev. 2011, 111, 2626–2704.
- 4For reviews, see:
- 4aC. Diner, K. J. Szabo, J. Am. Chem. Soc. 2017, 139, 2–14;
- 4bT. R. Ramadhar, R. A. Batey, Synthesis 2011, 1321–1346;
- 4cH.-X. Huo, J. R. Duvall, M.-Y. Huang, R. Hong, Org. Chem. Front. 2014, 1, 303–320; for selected examples, see:
- 4dM. Fujita, T. Nagano, U. Schneider, T. Hamada, C. Ogawa, S. Kobayashi, J. Am. Chem. Soc. 2008, 130, 2914–2915;
- 4eS. J. Jonker, C. Diner, G. Schulz, H. Iwamoto, L. Eriksson, K. Szabó, Chem. Commun. 2018, 54, 12852–12855;
- 4fR. J. Morrison, A. H. Hoveyda, Angew. Chem. Int. Ed. 2018, 57, 11654–11661; Angew. Chem. 2018, 130, 11828–11835;
- 4gH. Jang, F. Romiti, S. Torker, A. H. Hoveyda, Nat. Chem. 2017, 9, 1269–1275;
- 4hF. W. van der Mei, H. Miyamoto, D. L. Silverio, A. H. Hoveyda, Angew. Chem. Int. Ed. 2016, 55, 4701–4706; Angew. Chem. 2016, 128, 4779–4784;
- 4iD. L. Silverio, S. Torker, T. Pilyugina, E. M. Vieira, M. L. Snapper, F. Haeffner, A. H. Hoveyda, Nature 2013, 494, 216–221;
- 4jE. M. Vieira, M. L. Snapper, A. H. Hoveyda, J. Am. Chem. Soc. 2011, 133, 3332–3335;
- 4kS. Lou, P. N. Moquist, S. E. Schaus, J. Am. Chem. Soc. 2007, 129, 15398–15404;
- 4lK. Yeung, R. E. Ruscoe, J. Rae, A. P. Pulis, D. J. Procter, Angew. Chem. Int. Ed. 2016, 55, 11912–11916; Angew. Chem. 2016, 128, 12091–12095;
- 4mB. Alam, C. Diner, S. Jonker, L. Eriksson, K. J. Szabo, Angew. Chem. Int. Ed. 2016, 55, 14417–14421; Angew. Chem. 2016, 128, 14629–14633;
- 4nP. Zhang, I. A. Roundtree, J. P. Morken, Org. Lett. 2012, 14, 1416–1419;
- 4oG. Dutheuil, N. Selander, K. J. Szabo, V. K. Aggarwal, Synthesis 2008, 14, 2293–2297;
- 4pM. Murata, S. Watanabe, Y. Masuda, Tetrahedron Lett. 2000, 41, 5877–5880;
- 4qT. Ishiyama, T.-A. Ahiko, N. Miyaura, Tetrahedron Lett. 1996, 37, 6889–6892.
- 5
- 5aB. M. Trost, J.-P. Surivet, Angew. Chem. Int. Ed. 2000, 39, 3122–3124;
10.1002/1521-3773(20000901)39:17<3122::AID-ANIE3122>3.0.CO;2-8 CAS PubMed Web of Science® Google ScholarAngew. Chem. 2000, 112, 3252–3254;
- 5bB. M. Trost, J.-P. Surivet, J. Am. Chem. Soc. 2000, 122, 6291–6292.
- 6K. Ohmatsu, M. Ito, T. Kunieda, T. Ooi, Nat. Chem. 2012, 4, 473–477.
- 7X.-F. Yang, W.-H. Yu, C.-H. Ding, Q.-P. Ding, S.-L. Wan, X.-L. Hou, L.-X. Dai, P.-J. Wang, J. Org. Chem. 2013, 78, 6503–6509.
- 8M. Nakoji, T. Kanayama, T. Okino, Y. Takemoto, J. Org. Chem. 2002, 67, 7418–7423.
- 9T. Kanayama, K. Yoshida, H. Miyabe, Y. Takemoto, Angew. Chem. Int. Ed. 2003, 42, 2054–2056; Angew. Chem. 2003, 115, 2100–2102.
- 10
- 10aX. Huo, J. Zhang, J. Fu, R. He, W. Zhang, J. Am. Chem. Soc. 2018, 140, 2080–2084;
- 10bX. Huo, R. He, J. Fu, J. Zhang, G. Yang, W. Zhang, J. Am. Chem. Soc. 2017, 139, 9819–9822.
- 11
- 11aJ. Liu, C.-G. Cao, H.-B. Sun, X. Zhang, D. Niu, J. Am. Chem. Soc. 2016, 138, 13103–13106;
- 11bL. Wan, L. Tian, J. Liu, D. Niu, Synlett 2017, 28, 2051–2056.
- 12For a recent review on catalyst-controlled stereodivergence, see:
- 12aS. Krautwald, E. M. Carreira, J. Am. Chem. Soc. 2017, 139, 5627–5639; for selected additional examples, see:
- 12bF. A. Cruz, V. M. Dong, J. Am. Chem. Soc. 2017, 139, 1029–1032;
- 12cS. Kassem, A. T. Lee, D. A. Leigh, V. Marcos, L. I. Palmer, S. Pisano, Nature 2017, 549, 374–378;
- 12dT. Sandmeier, S. Krautwald, H. F. Zipfel, E. M. Carreira, Angew. Chem. Int. Ed. 2015, 54, 14363–14367; Angew. Chem. 2015, 127, 14571–14575;
- 12eS. Krautwald, M. A. Schafroth, D. Sarlah, E. M. Carreira, J. Am. Chem. Soc. 2014, 136, 3020–3023;
- 12fS. Krautwald, D. Sarlah, M. A. Schafroth, E. M. Carreira, Science 2013, 340, 1065–1068.
- 13For an excellent modular stereodivergent approach to enantioenriched allylic amines bearing vicinal stereogenic centers via three sequential catalyst-controlled reactions, see:
- 13aP. Tosatti, A. J. Campbell, D. House, A. Nelson, S. P. Marsden, J. Org. Chem. 2011, 76, 5495–5510.
- 14
- 14aK. J. Schwarz, J. L. Amos, J. C. Klein, D. T. Do, T. N. Snaddon, J. Am. Chem. Soc. 2016, 138, 5214–5217;
- 14bK. J. Schwarz, C. M. Pearson, G. A. Cintron-Rosado, P. Liu, T. N. Snaddon, Angew. Chem. Int. Ed. 2018, 57, 7800–7803; Angew. Chem. 2018, 130, 7926–7929;
- 14cJ. W. B. Fyfe, O. M. Kabia, C. M. Pearson, T. N. Snaddon, Tetrahedron 2018, 74, 5383–5391;
- 14dK. J. Schwarz, C. Yang, J. W. B. Fyfe, T. N. Snaddon, Angew. Chem. Int. Ed. 2018, 57, 12102–12105; Angew. Chem. 2018, 130, 12278–12281;
- 14eW. R. Scaggs, T. N. Snaddon, Chem. Eur. J. 2018, 24, 14378–14381;
- 14fL. Hutchings-Goetz, C. Yang, T. N. Snaddon, ACS Catal. 2018, 8, 10537–10544;
- 14gW. Rush Scaggs, T. D. Scaggs, T. N. Snaddon, Org. Biomol. Chem. 2019, 17, 1787–1790.
- 15For other examples of C1-ammonium enolates being employed in conjunction with transition-metal catalysis, see:
- 15aS. S. Spoehrle, T. H. West, J. E. Taylor, A. M. Z. Slawin, A. D. Smith, J. Am. Chem. Soc. 2017, 139, 11895–11902;
- 15bZ. Jiang, J. J. Beiger, J. F. Hartwig, J. Am. Chem. Soc. 2017, 139, 87–90;
- 15cX. Lu, L. Ge, C. Cheng, J. Chen, W. Cao, X. Wu, Chem. Eur. J. 2017, 23, 7689–7693;
- 15dJ. Song, Z.-J. Zhang, L.-Z. Gong, Angew. Chem. Int. Ed. 2017, 56, 5212–5216; Angew. Chem. 2017, 129, 5296–5300;
- 15eJ. Song, Z.-J. Zhang, S.-S. Chen, T. Fan, L.-Z. Gong, J. Am. Chem. Soc. 2018, 140, 3177–3180.
- 16For reviews on C1-ammonium enolate generation and reactivity, see:
- 16aW. C. Hartley, T. J. C. O'Riordan, A. D. Smith, Synthesis 2017, 49, 3303–3310;
- 16bL. C. Morrill, A. D. Smith, Chem. Soc. Rev. 2014, 43, 6214–6226;
- 16cM. J. Gaunt, C. C. Johansson, Chem. Rev. 2007, 107, 5596–5605;
- 16dD. H. Paull, A. Weatherwax, T. Lectka, Tetrahedron 2009, 65, 6771–6803;
- 16eS. France, D. J. Guerin, S. J. Miller, T. Lectka, Chem. Rev. 2003, 103, 2985–3012.
- 17For computation supporting the structure of benzotetramisole-derived C1-ammonium enolates, including a discussion of stereocontrol, see:
- 17aT. H. West, D. M. Walden, J. E. Taylor, A. C. Brueckner, R. C. Johnstone, P. H.-Y. Cheong, G. C. Lloyd-Jones, A. D. Smith, J. Am. Chem. Soc. 2017, 139, 4366–4375;
- 17bE. R. T. Robinson, D. M. Waldon, C. Fallan, M. D. Greenhalgh, P. H.-Y. Cheong, A. D. Smith, Chem. Sci. 2016, 7, 6919–6927.
- 18For reviews concerning catalyzed enantioselective reactions proceeding via π(allyl)Pd electrophiles, see:
- 18aJ. Tsuji, Tetrahedron 2015, 71, 6330–6348;
- 18bB. M. Trost, Tetrahedron 2015, 71, 5708–5733;
- 18cJ. D. Weaver, A. Recio III, A. J. Grenning, J. A. Tunge, Chem. Rev. 2011, 111, 1846–1913;
- 18dB. M. Trost, M. R. Machacek, A. P. Aponick, Acc. Chem. Res. 2006, 39, 747–760;
- 18eB. M. Trost, M. L. Crawley, Chem. Rev. 2003, 103, 2921–2944;
- 18fB. M. Trost, D. L. Van Vranken, Chem. Rev. 1996, 96, 395–422.
- 19For computation concerning benzotetramisole-containing C1-ammonium enolates reacting with cationic π(allyl)PdII electrophiles through an outer-sphere mechanism, see Ref. [14b].
- 20Pentafluorophenyl esters are established acyl donors in peptide bond formation; for examples, see:
- 20aL. M. Gayo, M. J. Suto, Tetrahedron Lett. 1996, 37, 4915–4918;
- 20bM. Green, J. Berman, Tetrahedron Lett. 1990, 31, 5851–5852;
- 20cE. Atherton, L. R. Cameron, R. C. Sheppard, Tetrahedron 1988, 44, 843–857;
- 20dL. Kisfaludy, J. Roberts, R. Johnson, G. L. Mayers, J. Kovacs, J. Org. Chem. 1970, 35, 3563–3565.
- 21L. Kurti, B. Czakó, Strategic Applications of Named Reactions in Organic Synthesis, Elsevier, Amsterdam, 2005.
- 22There are isolated reports of the Hofmann rearrangement being used to prepare homoallylic amines; however, none of these constitutes a general method for the regio- and stereodivergent synthesis of enantioenriched homoallylic amines; see:
- 22aK. G. M. Kou, S. Kulyk, C. J. Marth, J. C. Lee, N. A. Doering, B. X. Li, G. M. Galego, T. P. Lebold, R. Sarpong, J. Am. Chem. Soc. 2017, 139, 13882–13896;
- 22bC. J. Marth, G. M. Galego, J. C. Lee, T. P. Lebold, S. Kulyk, K. G. M. Kou, J. Qin, R. Lilien, R. Sarpong, Nature 2015, 528, 493–498;
- 22cM. Ito, Y. Kondon, H. Nambu, M. Anada, K. Takeda, S. Hashimoto, Tetrahedron Lett. 2015, 56, 1397–1400;
- 22dS. Umezaki, S. Yokoshima, T. Fukuyama, Org. Lett. 2013, 15, 4230–4233;
- 22eC. Sosale, M. V. Rao, Beilstein J. Org. Chem. 2012, 8, 1393–1399;
- 22fJ.-H. Ye, Y. Huang, R.-Y. Chen, Org. Prep. Proced. Int. 2003, 35, 429–432;
- 22gK. A. Newlander, J. F. Callahan, M. L. Moore, T. A. Tomaszek, Jr., W. F. Huffmann, J. Med. Chem. 1993, 36, 2321–2331.
- 23For an excellent recent example of stereodivergence via the addition of stereodefined E- or Z-configured allylboronic acid nucleophiles to indoles and dihydroisoquinolines, see Ref. [4 m].
- 24For a recent review, see: A. Yoshimura, V. V. Zhdankin, Chem. Rev. 2016, 116, 3328–3435.
- 25
- 25aA. A. Zagulyaeva, C. T. Banek, M. S. Yusubov, V. V. Zhdankin, Org. Lett. 2010, 12, 4644–4647;
- 25bP. Liu, Z. Wang, X. Hu, Eur. J. Org. Chem. 2012, 1994–2000;
- 25cA. Yoshimura, K. R. Middleton, M. W. Luedtke, C. Zhu, V. V. Zhdankin, J. Org. Chem. 2012, 77, 11399–11404;
- 25dA. Yoshimura, M. W. Luedtke, V. V. Zhdankin, J. Org. Chem. 2012, 77, 2087–2091.
- 26
- 26aP. Liu, X. Yang, V. B. Birman, K. N. Houk, Org. Lett. 2012, 14, 3288–3291;
- 26bV. D. Bumbu, V. B. Birman, J. Am. Chem. Soc. 2011, 133, 13902–13905;
- 26cX. Yang, G. Lu, V. B. Birman, Org. Lett. 2010, 12, 892–895;
- 26dV. B. Birman, X. Li, Org. Lett. 2006, 8, 1351–1354; for a discussion of isothioureas as enantioselective Lewis base catalysts, see:
- 26eJ. Merad, J.-M. Pons, O. Chuzel, C. Bressy, Eur. J. Org. Chem. 2016, 5589–5560.
- 27N. C. Bruno, M. T. Tudge, S. L. Buchwald, Chem. Sci. 2013, 4, 916–920.
- 28P. J. Kocienski, Protecting Groups, 3rd ed., Thieme, Stuttgart, 2005.
10.1055/b-003-108603 Google Scholar
- 29W. Li, Y. Han, B. Li, C. Liu, Z. Bo, J. Polym. Sci. Part A 2008, 46, 4556–4563.
- 30For access to related products by vinylogous Mannich reactions, see: M. Sickert, C. Schneider, Angew. Chem. Int. Ed. 2008, 47, 3631–3634; Angew. Chem. 2008, 120, 3687–3690.
- 31For reviews on Pd-catalyzed cross-couplings of organosilicon compounds, see:
- 31aY. Nakao, T. Hiyama, Chem. Soc. Rev. 2011, 40, 4893–4901;
- 31bS. E. Denmark, J. Liu, Angew. Chem. Int. Ed. 2010, 49, 2978–2986; Angew. Chem. 2010, 122, 3040–3049;
- 31cH. F. Sore, W. R. Galloway, D. R. Spring, Chem. Soc. Rev. 2012, 41, 1845–1866.
- 32
- 32aB. M. Trost, M. R. Machacek, Z. T. Ball, Org. Lett. 2003, 5, 1895–1898;
- 32bM. G. McLaughlin, C. A. McAdam, M. J. Cook, Org. Lett. 2015, 17, 10–13.
- 33R. Kalshetti, V. Venkataramasubramanian, S. Kamble, A. Sudalai, Tetrahedron Lett. 2016, 57, 1053–1055.
- 34
- 34aJ. D. Huber, J. L. Leighton, J. Am. Chem. Soc. 2007, 129, 14552–14553;
- 34bJ. D. Huber, N. R. Perl, J. L. Leighton, Angew. Chem. Int. Ed. 2008, 47, 3037–3039; Angew. Chem. 2008, 120, 3079–3081.
- 35D. Sun, Z. Li, Y. Rew, M. Gribble, M. D. Bartberger, H. P. Beck, J. Canon, A. Chen, X. Chen, D. Chow, J. Deignan, J. Duquette, J. Eksterowicz, B. Fisher, B. M. Fox, J. Fu, A. Z. Gonzalez, F. Gonzalex-Lopez De Turiso, J. B. Houze, X. Huang, M. Jiang, L. Jin, F. Kayser, J. Liu, M.-C. Lo, A. M. Long, B. Lucas, L. R. McGee, J. McIntosh, J. Mihalic, J. D. Oliner, T. Osgood, M. L. Peterson, P. Roveto, A. Y. Saiki, P. Shaffer, M. Toteva, Y. Wang, Y. C. Wang, S. Wortman, P. Yakowec, X. Yan, Q. Ye, D. Yu, M. Yu, X. Zhao, J. Zhou, J. Zhu, S. H. Olsen, J. C. Medina, J. Med. Chem. 2014, 57, 1454–1472.
- 36Our efforts to incorporate analogous Curtius and Lossen rearrangements (via acyl azides and hydroxamic acids, respectively) into a similar general one-pot procedure have been unsuccessful.