Enantioselective Palladium-Catalyzed Cross-Coupling of α-Bromo Carboxamides and Aryl Boronic Acids
Bowen Li
State Key Laboratory of Bio-Organic and Natural Products Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, 345 Ling Ling Rd, Shanghai, 200032 China
Search for more papers by this authorTiejun Li
State Key Laboratory of Bio-Organic and Natural Products Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, 345 Ling Ling Rd, Shanghai, 200032 China
Search for more papers by this authorMuinat A. Aliyu
State Key Laboratory of Bio-Organic and Natural Products Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, 345 Ling Ling Rd, Shanghai, 200032 China
Search for more papers by this authorProf. Dr. Zhen Hua Li
Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Department of Chemistry, Fudan University, Handan Road 220, Shanghai, 200438 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Wenjun Tang
State Key Laboratory of Bio-Organic and Natural Products Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, 345 Ling Ling Rd, Shanghai, 200032 China
Search for more papers by this authorBowen Li
State Key Laboratory of Bio-Organic and Natural Products Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, 345 Ling Ling Rd, Shanghai, 200032 China
Search for more papers by this authorTiejun Li
State Key Laboratory of Bio-Organic and Natural Products Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, 345 Ling Ling Rd, Shanghai, 200032 China
Search for more papers by this authorMuinat A. Aliyu
State Key Laboratory of Bio-Organic and Natural Products Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, 345 Ling Ling Rd, Shanghai, 200032 China
Search for more papers by this authorProf. Dr. Zhen Hua Li
Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Department of Chemistry, Fudan University, Handan Road 220, Shanghai, 200438 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Wenjun Tang
State Key Laboratory of Bio-Organic and Natural Products Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, 345 Ling Ling Rd, Shanghai, 200032 China
Search for more papers by this authorGraphical Abstract
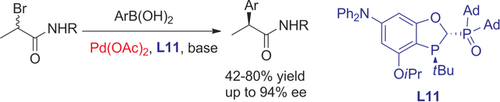
A palladium catalyst loading as low as 0.5 mol % enables the cross-coupling of α-bromo carboxamides and aryl boronic acids, generating a series of chiral α-aryl carboxamides in good yields and excellent enantioselectivities. The development of a chiral P,P=O ligand was critical in suppressing the formation of biaryl side products.
Abstract
We herein report an enantioselective palladium-catalyzed cross-coupling between α-bromo carboxamides and aryl boronic acids, generating a series of chiral α-aryl carboxamides in good yields and excellent enantioselectivities. The development of a chiral P,P=O ligand was critical in overcoming the second transmetalation issue and allows the first asymmetric palladium-catalyzed coupling of α-bromo carbonyl compounds.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201905174-sup-0001-misc_information.pdf25.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews on enantioselective and enantiospecific aryl–alkyl couplings:
- 1aA. H. Cherney, N. T. Kadunce, S. E. Reisman, Chem. Rev. 2015, 115, 9587;
- 1bS. Z. Tasker, E. A. Standley, T. F. Jamison, Nature 2014, 509, 299.
- 2For transition-metal-free cross-couplings, see:
- 2aC. Li, Y. Zhang, Q. Sun, T. Gu, H. Peng, W. Tang, J. Am. Chem. Soc. 2016, 138, 10774;
- 2bD. Tian, C. Li, G. Gu, H. Peng, X. Zhang, W. Tang, Angew. Chem. Int. Ed. 2018, 57, 7176; Angew. Chem. 2018, 130, 7294.
- 3
- 3aX. Dai, N. A. Strotman, G. C. Fu, J. Am. Chem. Soc. 2008, 130, 3302;
- 3bP. M. Lundin, J. Esquivias, G. C. Fu, Angew. Chem. Int. Ed. 2009, 48, 154; Angew. Chem. 2009, 121, 160;
- 3cP. M. Lundin, G. C. Fu, J. Am. Chem. Soc. 2010, 132, 11027;
- 3dS. Lou, G. C. Fu, J. Am. Chem. Soc. 2010, 132, 1264;
- 3eQ. M. Kainz, C. D. Matier, A. Bartoszewicz, S. L. Zultanski, J. C. Peters, G. C. Fu, Science 2016, 351, 681;
- 3fY. Liang, G. C. Fu, J. Am. Chem. Soc. 2014, 136, 5520.
- 4
- 4aJ. Mao, F. Liu, M. Wang, L. Wu, B. Zheng, S. Liu, J. Zhong, Q. Bian, P. J. Walsh, J. Am. Chem. Soc. 2014, 136, 17662;
- 4bF. Liu, J. Zhong, Y. Zhou, Z. Gao, P. J. Walsh, W. Wang, S. Ma, S. Hou, S. Liu, M. Wang, M. Wang, Q. Bian, Chem. Eur. J. 2018, 24, 2059.
- 5
- 5aM. Jin, L. Adak, M. Nakamura, J. Am. Chem. Soc. 2015, 137, 7128;
- 5bT. Iwamoto, C. Okuzono, L. Adak, M. Jin, M. Nakamura, Chem. Commun. 2019, 55, 1128.
- 6C. Liu, C. He, W. Shi, M. Chen, A. Lei, Org. Lett. 2007, 9, 5601.
- 7
- 7aQ. Liu, Y. Lan, J. Liu, G. Li, Y.-D. Wu, A. Lei, J. Am. Chem. Soc. 2009, 131, 10201;
- 7bA. Lei, X. Zhang, Tetrahedron Lett. 2002, 43, 2525;
- 7cJ. Wang, G. Meng, K. Xie, L. Li, H. Sun, Z. Huang, ACS Catal. 2017, 7, 7421.
- 8For reviews on ligand development and cross-coupling, see:
- 8aG. Xu, C. H. Senanayake, W. Tang, Acc. Chem. Res. 2019, 52, 1101;
- 8bH. Yang, W. Tang, Chem. Rec. 2019, https://doi.org/10.1002/tcr.201900003;
- 8cC. Li, D. Chen, W. Tang, Synlett 2016, 27, 2183.
- 9
- 9aC. Li, T. Chen, B. Li, G. Xiao, W. Tang, Angew. Chem. Int. Ed. 2015, 54, 3792; Angew. Chem. 2015, 127, 3863;
- 9bT. Si, B. Li, W. Xiong, B. Xu, W. Tang, Org. Biomol. Chem. 2017, 15, 9903.
- 10L. Chu, X.-C. Wang, C. E. Moore, A. L. Rheingold, J.-Q. Yu, J. Am. Chem. Soc. 2013, 135, 16344.
- 11The electronic properties of the N-CH(2-MeOC6H4)2 group also have an important influence on both the yield and the ee as the number and position of the methoxy groups have a significant effect; see entries 26 and 27.
- 12R. Omar-Amrani, A. Thomas, E. Brenner, R. Schneider, Y. Fort, Org. Lett. 2003, 5, 2311.
- 13J. M. Khanna, N. Anand, J. Med. Chem. 1967, 10, 944.
- 14
- 14aM. F. Landoni, A. Soraci, Curr. Drug Metab. 2001, 2, 37;
- 14bS.-F. Zhu, Y.-B. Yu, S. Li, L.-X. Wang, Q.-L. Zhou, Angew. Chem. Int. Ed. 2012, 51, 8872; Angew. Chem. 2012, 124, 9002;
- 14cI. Shiina, K. Nakata, K. Ono, Y.-S. Onda, M. Itagaki, J. Am. Chem. Soc. 2010, 132, 11629;
- 14dA. Bigot, A. E. Williamson, M. J. Gaunt, J. Am. Chem. Soc. 2011, 133, 13778;
- 14eX. Chen, J. Z. M. Fong, J. Xu, C. Mou, Y. Lu, S. Yang, B.-A. Song, Y. R. Chi, J. Am. Chem. Soc. 2016, 138, 7212.
- 15
- 15aM. T. Reetz, S. Prasad, J. D. Carballeira, Y. Gumulya, M. Bocola, J. Am. Chem. Soc. 2010, 132, 9144;
- 15bC. E. Stivala, A. Zakarian, J. Am. Chem. Soc. 2011, 133, 11936;
- 15cG. J. Harkness, M. L. Clarke, Eur. J. Org. Chem. 2017, 4859.