Iron(II)-Based Metalloradical Activation: Switch from Traditional Click Chemistry to Denitrogenative Annulation
Satyajit Roy
Division of Molecular Synthesis & Drug Discovery, Centre of Bio-Medical Research (CBMR), SGPGIMS Campus, Raebareli Road, Lucknow, 226014 U.P., India
These authors contributed equally to this work.
Search for more papers by this authorHillol Khatua
Division of Molecular Synthesis & Drug Discovery, Centre of Bio-Medical Research (CBMR), SGPGIMS Campus, Raebareli Road, Lucknow, 226014 U.P., India
These authors contributed equally to this work.
Search for more papers by this authorSandip Kumar Das
Division of Molecular Synthesis & Drug Discovery, Centre of Bio-Medical Research (CBMR), SGPGIMS Campus, Raebareli Road, Lucknow, 226014 U.P., India
Search for more papers by this authorCorresponding Author
Prof. Dr. Buddhadeb Chattopadhyay
Division of Molecular Synthesis & Drug Discovery, Centre of Bio-Medical Research (CBMR), SGPGIMS Campus, Raebareli Road, Lucknow, 226014 U.P., India
Search for more papers by this authorSatyajit Roy
Division of Molecular Synthesis & Drug Discovery, Centre of Bio-Medical Research (CBMR), SGPGIMS Campus, Raebareli Road, Lucknow, 226014 U.P., India
These authors contributed equally to this work.
Search for more papers by this authorHillol Khatua
Division of Molecular Synthesis & Drug Discovery, Centre of Bio-Medical Research (CBMR), SGPGIMS Campus, Raebareli Road, Lucknow, 226014 U.P., India
These authors contributed equally to this work.
Search for more papers by this authorSandip Kumar Das
Division of Molecular Synthesis & Drug Discovery, Centre of Bio-Medical Research (CBMR), SGPGIMS Campus, Raebareli Road, Lucknow, 226014 U.P., India
Search for more papers by this authorCorresponding Author
Prof. Dr. Buddhadeb Chattopadhyay
Division of Molecular Synthesis & Drug Discovery, Centre of Bio-Medical Research (CBMR), SGPGIMS Campus, Raebareli Road, Lucknow, 226014 U.P., India
Search for more papers by this authorGraphical Abstract
Abstract
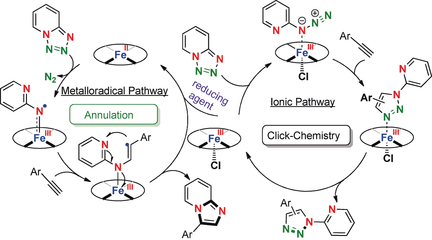
A unique concept for the intermolecular denitrogenative annulation of 1,2,3,4-tetrazoles and alkynes was discovered by using a catalytic amount of Fe(TPP)Cl and Zn dust. The reaction precludes the traditional, more favored click reaction between an organic azide and alkynes, and instead proceeds by an unprecedented metalloradical activation. The method is anticipated to advance access to the construction of important basic nitrogen heterocycles, which will in turn enable discoveries of new drug candidates.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201904702-sup-0001-misc_information.pdf7.8 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected few examples of cyclopropanation, see:
- 1aY. Wang, X. Wen, X. Cui, L. Wojtas, X. P. Zhang, J. Am. Chem. Soc. 2017, 139, 1049;
- 1bX. Xu, Y. Wang, X. Cui, L. Wojtasb, X. P. Zhang, Chem. Sci. 2017, 8, 4347;
- 1cS. Zhu, X. Xu, J. A. Perman, X. P. Zhang, J. Am. Chem. Soc. 2010, 132, 12796;
- 1dS. Zhu, J. A. Perman, X. P. Zhang, Angew. Chem. Int. Ed. 2008, 47, 8460; Angew. Chem. 2008, 120, 8588;
- 1eS. Zhu, J. V. Ruppel, H. Lu, L. Wojtas, X. P. Zhang, J. Am. Chem. Soc. 2008, 130, 5042;
- 1fY. Chen, J. V. Ruppel, X. P. Zhang, J. Am. Chem. Soc. 2007, 129, 12074;
- 1gY. Chen, K. B. Fields, X. P. Zhang, J. Am. Chem. Soc. 2004, 126, 14718.
- 2For selected few examples of heterocyclization and carbocyclization, except cyclopropanation, see:
- 2aC. te Grotenhuis, N. van den Heuvel, J. I. van der Vlugt, B. de Bruin, Angew. Chem. Int. Ed. 2018, 57, 140; Angew. Chem. 2018, 130, 146;
- 2bH. L. Jiang, K. Lang, H. J. Lu, L. Wojtas, X. P. Zhang, J. Am. Chem. Soc. 2017, 139, 9164;
- 2cC. te Grotenhuis, B. G. Das, P. F. Kuijpers, W. Hageman, M. Trouwborst, B. de Bruin, Chem. Sci. 2017, 8, 8221;
- 2dX. Cui, X. Xu, L. M. Jin, L. Wojtas, X. P. Zhang, Chem. Sci. 2015, 6, 1219;
- 2eN. D. Paul, S. Mandal, M. Otte, X. Cui, X. P. Zhang, B. de Bruin, J. Am. Chem. Soc. 2014, 136, 1090;
- 2fB. G. Das, A. Chirila, M. Tromp, J. N. H. Reek, B. de Bruin, J. Am. Chem. Soc. 2014, 136, 8968;
- 2gX. Cui, X. Xu, L. Wojtas, M. M. Kim, X. P. Zhang, J. Am. Chem. Soc. 2012, 134, 19981;
- 2hX. Xu, H. Lu, J. V. Ruppel, X. Cui, S. L. de Mesa, L. Wojtas, X. P. Zhang, J. Am. Chem. Soc. 2011, 133, 15292.
- 3
- 3aH. Lu, H. Jiang, L. Wojtas, X. P. Zhang, Angew. Chem. Int. Ed. 2010, 49, 10192; Angew. Chem. 2010, 122, 10390;
- 3bH. Lu, V. Subbarayan, J. Tao, X. P. Zhang, Organometallics 2010, 29, 389;
- 3cH. Lu, J. Tao, J. E. Jones, L. Wojtas, X. P. Zhang, Org. Lett. 2010, 12, 1248;
- 3dH. Lu, H. Jiang, Y. Hu, L. Wojtas, X. P. Zhang, Chem. Sci. 2011, 2, 2361;
- 3eH. Lu, Y. Hu, H. Jiang, L. Wojtas, X. P. Zhang, Org. Lett. 2012, 14, 5158;
- 3fL.-M. Jin, H. Lu, Y. Cui, C. L. Lizardi, T. N. Arzua, L. Wojtas, X. Cui, X. P. Zhang, Chem. Sci. 2014, 5, 2422;
- 3gH. Lu, C. Li, H. Jiang, C. L. Lizardi, X. P. Zhang, Angew. Chem. Int. Ed. 2014, 53, 7028; Angew. Chem. 2014, 126, 7148;
- 3hO. Villanueva, N. M. Weldy, S. B. Blakey, C. E. MacBeth, Chem. Sci. 2015, 6, 6672;
- 3iH. Lu, K. Lang, H. Jiang, L. Wojtas, X. P. Zhang, Chem. Sci. 2016, 7, 6934;
- 3jP. F. Kuijpers, M. J. Tiekink, W. B. Breukelaar, D. L. J. Broere, N. P. van Leest, J. I. van der Vlugt, J. N. H. Reek, B. de Bruin, Chem. Eur. J. 2017, 23, 7945;
- 3kZ.-Y. Gu, Y. Liu, F. Wang, X. Bao, S.-Y. Wang, S.-J. Ji, ACS Catal. 2017, 7, 3893;
- 3lY.-D. Du, Z.-J. Xu, C.-Y. Zhou, C.-M. Che, Org. Lett. 2019, 21, 895.
- 4
- 4aJ. V. Ruppel, J. E. Jones, C. A. Huff, R. M. Kamble, Y. Chen, X. P. Zhang, Org. Lett. 2008, 10, 1995;
- 4bV. Subbarayan, J. V. Ruppel, S. Zhu, J. A. Perman, X. P. Zhang, Chem. Commun. 2009, 4266;
- 4cL.-M. Jin, X. Xu, H. Lu, X. Cui, L. Wojtas, X. P. Zhang, Angew. Chem. Int. Ed. 2013, 52, 5309; Angew. Chem. 2013, 125, 5417;
- 4dH. Jiang, K. Lang, H. Lu, L. Wojtas, X. P. Zhang, Angew. Chem. Int. Ed. 2016, 55, 11604; Angew. Chem. 2016, 128, 11776.
- 5For the first report of denitrogenative annulation by metalloradical activation mechanism, see: S. Roy, S. K. Das, B. Chattopadhyay, Angew. Chem. Int. Ed. 2018, 57, 2238; Angew. Chem. 2018, 130, 2260.
- 6B. Chattopadhyay, C. I. Rivera Vera, S. Chuprakov, V. Gevorgyan, Org. Lett. 2010, 12, 2166.
- 7For selected pioneering examples, see:
- 7aR. Harder, C. Wentrup, J. Am. Chem. Soc. 1976, 98, 1259;
- 7bC. Wentrup, H.-W. Winter, J. Am. Chem. Soc. 1980, 102, 6159;
- 7cR. A. Evans, M. W. Wong, C. Wentrup, J. Am. Chem. Soc. 1996, 118, 4009;
- 7dC. Wentrup, M. Kuzaj, H. Lüerssen, Angew. Chem. Int. Ed. Engl. 1986, 25, 480; Angew. Chem. 1986, 98, 476;
- 7eC. Wentrup, Tetrahedron 1970, 27, 367;
- 7fD. Kvaskoff, M. Vosswinkel, C. Wentrup, J. Am. Chem. Soc. 2011, 133, 5413.
- 8For leading reviews on denitrogenative annulations of 1,2,3-triazoles using transition-metal catalysts, see:
- 8aB. Chattopadhyay, V. Gevorgyan, Angew. Chem. Int. Ed. 2012, 51, 862; Angew. Chem. 2012, 124, 886;
- 8bA. V. Gulevich, V. Gevorgyan, Angew. Chem. Int. Ed. 2013, 52, 1371; Angew. Chem. 2013, 125, 1411;
- 8cH. M. L. Davies, J. S. Alford, Chem. Soc. Rev. 2014, 43, 5151.
- 9For the first report of intramolecular annulation of 1,2,3,4-tetrazole via a metal-nitrene, see:
- 9aS. K. Das, S. Roy, H. Khatua, B. Chattopadhyay, J. Am. Chem. Soc. 2018, 140, 8429;
- 9bFor the first denitrogenative annulation with monocyclic 1,2,3,4-tetrazoles via a metal-carbene intermediate, see: T. Nakamuro, K. Hagiwara, T. Miura, M. Murakami, Angew. Chem. Int. Ed. 2018, 57, 5497; Angew. Chem. 2018, 130, 5595.
- 10Notably, this is the first example of metalloradical activation of organic azides for radical annulation with alkynes.
- 11B. J. Stokes, H. Dong, B. E. Leslie, A. L. Pumphrey, T. G. Driver, J. Am. Chem. Soc. 2007, 129, 7500.
- 12For lower reactivity of 2-pyridyl diazo-compounds in Rh-catalyzed reactions, see: H. M. L. Davies, R. J. Townsend, J. Org. Chem. 2001, 66, 6595.
- 13C. K. Prier, R. Zhang, A. R. Buller, S. Brinkmann-Chen, F. H. Arnold, Nat. Chem. 2017, 9, 629.
- 14The possibility of the annulation product (3 a) from the click product (3 a′) was discard by the control experiment using the independently synthesized click product (3 a′) under the optimized annulation conditions.
- 15Importantly, performing a reaction at 130 °C temperature in the presence of Fe(TPP)Cl and Zn dust, we observed only the annulation product. No click reaction occurred, demonstrating that our reaction conditions are highly effective even at high reaction temperature.
- 16For Fe-catalyzed nitrogen heterocycle synthesis, see: E. T. Hennessy, T. A. Betley, Science 2013, 340, 591.